PREM14C: Preliminary information statements relating to merger or acquisition
Published on January 16, 2026
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
__________________________________________
SCHEDULE 14C
__________________________________________
Information Statement Pursuant to Section 14(c) of the
Securities Exchange Act of 1934
Check the appropriate box:
|
☒ |
Preliminary Information Statement |
|
|
☐ |
Confidential, for Use of the Commission Only (as permitted by Rule 14c-5(d)(2)) |
|
|
☐ |
Definitive Information Statement |
Apimeds Pharmaceuticals US, Inc.
(Name of Registrant as Specified in its Charter)
Payment of Filing Fee (Check all boxes that apply):
|
☐ |
No fee required |
|
|
☐ |
Fee paid previously with preliminary materials |
|
|
☒ |
Fee computed on table in exhibit required by Item 25(b) of Schedule 14A (17 CFR 240.14a-101) per Item 1 of this Schedule and Exchange Act Rules 14c-5(g) and 0-11. |
PRELIMINARY INFORMATION STATEMENT — SUBJECT TO COMPLETION
APIMEDS PHARMACEUTICALS US, INC.
100 Matawan Rd, Suite 325
Matawan, New Jersey 07747
NOTICE OF STOCKHOLDER ACTION BY WRITTEN CONSENT
WE ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY
THIS IS NOT A NOTICE OF A MEETING OF STOCKHOLDERS AND NO STOCKHOLDERS’ MEETING WILL BE HELD TO CONSIDER ANY
MATTER DESCRIBED HEREIN. THIS INFORMATION STATEMENT IS BEING FURNISHED TO YOU SOLELY FOR THE PURPOSE OF
INFORMING YOU OF THE MATTERS DESCRIBED HEREIN.
Dear Stockholders:
This notice and information statement (the “Information Statement”) have been filed with the U.S. Securities and Exchange Commission (the “SEC”), and are being mailed or otherwise furnished to the stockholders of Apimeds Pharmaceuticals US, Inc., a Delaware corporation (the “Company”, “we”, “us”, or “our”), of record as of the close of business on [—], 2026 (the “Record Date”), pursuant to Section 14(c) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the rules and regulations prescribed thereunder. Only stockholders of record as of the Record Date are entitled to receive this Information Statement.
The purpose of this Information Statement is to notify our stockholders that on December [—], 2025, certain stockholders of the Company (the “Consenting Stockholders”), collectively holding approximately [—]% of the Company’s shares of common stock, par value $0.01 per share (the “Common Stock”), approved by written consent in lieu of a special meeting (the “Written Consent”), in accordance with Section 228 of the Delaware General Corporation Law (the “DGCL”) and Article [—] of the Company’s Amended and Restated Certificate of Incorporation, the following actions (the “Corporate Actions”):
1. The Preferred Stock Conversion and Issuance: the issuance of Company Common Stock upon the conversion (the “Preferred Stock Conversion”) of the Series A Convertible Preferred Stock, par value $0.01 per share of the Company (the “Preferred Stock”);
2. The Notes Conversion and Issuance: the issuance of Company Common Stock upon the conversion of the convertible notes (the “Notes Conversion”) issued by the Company pursuant to that certain Securities Purchase Agreement, entered into by the Company and certain institutional investors on December 1, 2025, and amended by that certain Amendment No. 1 to Securities Purchase Agreement on December 8, 2025 (the “SPA”);
3. The Reverse Stock Split: a 1-for-10 reverse stock split (the “Reverse Stock Split”) of the Company’s Common Stock, a change in the par value per share of the Company’s Common Stock from $0.01 to $0.001 (the “Change in Par Value”), and a corresponding amendment to the Company’s Amended and Restated Certificate of Incorporation (the “Charter”) to (i) authorize the Board of Directors to effect the Reverse Stock split and (ii) the Change in Par Value;
4. The 2024 Plan Share Increase: an amendment to the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan (the “2024 Plan”) to increase the number of shares of Common Stock issuable under the 2024 Plan to 2,096,679; and
5. The 2025 Equity Plan: the approval and adoption the Apimeds Pharmaceuticals US, Inc. 2025 Equity Incentive Plan (the “2025 Plan”).
The Written Consent was entered into and the Corporate Actions were approved by the Consenting Stockholders in connection with that certain Agreement and Plan of Merger (the “Merger Agreement”), attached as Annex A, which was entered into and closed on December 1, 2025, by the Company, Apimeds Merger Sub, Inc., a Delaware corporation (“Merger Sub”), MindWave Innovations Inc, a Delaware corporation (“MindWave”), Lokahi Therapeutics, Inc., a Nevada corporation (“Bio Sub”), and Erik Emerson, solely in his capacity as representative for the Bio Business (the “Bio Business Representative”). The transactions contemplated by the Merger Agreement are referred to herein as the “Transactions” and the closing of the Transactions is referred to herein as the “Closing”.
Pursuant to the terms and conditions of the Merger Agreement, immediately prior to the Closing, a certificate of merger (the “Certificate of Merger”) was filed with the Secretary of State of the State of Delaware (the “DE SOS”) (such time of the filing of the Certificate of Merger, the “Effective Time”), in accordance with the DGCL. Pursuant to the Certificate of Merger, Merger Sub was merged with and into MindWave (the “Merger”), with MindWave surviving the Merger as the surviving corporation (the “Surviving Corporation”). As a result of the Merger, MindWave became a direct wholly owned subsidiary of the Company. At the Effective Time, all of the property, rights, privileges, powers and franchises of MindWave and Merger Sub vested in the Surviving Corporation and all of the debts, liabilities and duties of MindWave and Merger Sub became the debts, liabilities and duties of the Surviving Corporation. The Closing occurred simultaneously with the execution and delivery of the Merger Agreement on the Closing Date.
At the Effective Time, (i) each share of MindWave common stock issued and outstanding immediately prior to the Effective Time shall be canceled and converted into the right to receive a portion of the Merger Consideration, consisting of shares of Company Preferred Stock; and (ii) each holder of such shares shall receive, for each share of MindWave Common Stock held immediately prior to the Effective Time, a pro rata portion of the Merger Consideration, allocated as follows: (A) a number of duly authorized, validly issued, fully paid and nonassessable shares of Company Common Stock, such that the aggregate number of shares of Company Common Stock issued to all holders of MindWave Common Stock shall equal 0% of the total number of shares of Company Common Stock issued and outstanding as of the date of the Merger Agreement (the “Common Stock Cap”), with each holder’s allocation rounded down to the nearest whole share; and (B) a number of duly authorized, validly issued, fully paid and nonassessable shares of Company Preferred Stock, such that, immediately following the Effective Time, the holders of MindWave Common Stock collectively hold, on an as-converted to Company Common Stock basis, 90.9% of the total issued and outstanding equity securities of the Company (exclusive of the Company Common Stock issued pursuant to clause (A) and calculated on a fully diluted basis). The shares of Company Common Stock and Company Preferred Stock issued to holders of MindWave Common Stock pursuant to the Merger Agreement, on an as-converted and fully diluted basis, shall collectively represent 90.9% of the equity capital of the Company as of the Closing. For purposes of the Merger Agreement, the shares of Company Common Stock, Company Preferred Stock, and MindWave Common Stock issued pursuant to the Merger Agreement are collectively referred to as the “Merger Consideration.”
Additional information regarding the Merger Agreement is contained in the Company’s Current Report on Form 8-K filed with the SEC on December 10, 2025.
The accompanying Information Statement is first being mailed on or about [—], 2026, to our stockholders of record as of the Record Date. If you were a stockholder of record on such date, you will receive one or more copies of the accompanying Information Statement.
In accordance with Rule 14c-2 of the Exchange Act, and the rules promulgated by the SEC, this Information Statement is being furnished to our stockholders solely for the purpose of notifying our stockholders of the Corporate Actions taken in the Written Consent, which will be effective on or about [—], 2026, twenty (20) calendar days after the accompanying Information Statement is sent or mailed to the stockholders of record of the Company as of the Record Date (the “Action Effective Time”). [Furthermore, notwithstanding the approval provided by the Consenting Stockholders, the board of directors of the Company (the “Board of Directors”) retains sole discretion to implement or abandon the Reverse Stock Split based on its determination of whether effecting a reverse stock split is advisable and in the best interests of the Company and its stockholders.]
You are urged to read the Information Statement in its entirety for a description of the actions taken by the Consenting Stockholders.
WE ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY. NO VOTE OR OTHER ACTION OF THE COMPANY’S STOCKHOLDERS IS REQUIRED IN CONNECTION WITH THE INFORMATION STATEMENT.
EXISTING STOCKHOLDERS WILL RETAIN THEIR EXISTING COMMON STOCK.
|
BY ORDER OF THE BOARD OF DIRECTORS, |
||
|
|
||
|
Vin Menon |
||
|
Chief Executive Officer |
||
|
[—], 2026 |
This Information Statement is dated [—], 2026 and is first being mailed to the Company’s stockholders on [—], 2026.
i

Apimeds Pharmaceuticals US, Inc.
100 Matawan Rd, Suite 325
Matawan, New Jersey 07747
information statement pursuant to section 14(c)
of the securities exchange act of 1934, AS AMENDED
WE ARE NOT ASKING YOU FOR A PROXY, YOU ARE REQUESTED NOT TO SEND US A PROXY AND ACCORDINGLY NO PROXY CARD HAS
BEEN ENCLOSED WITH THIS INFORMATION STATEMENT. EXISTING STOCKHOLDERS WILL RETAIN THEIR EXISTING COMMON STOCK
[—], 2026
ABOUT THIS INFORMATION STATEMENT
This notice and information statement (the “Information Statement”) have been filed with the U.S. Securities and Exchange Commission (the “SEC”), and are being mailed or otherwise furnished to the stockholders of Apimeds Pharmaceuticals US, Inc., a Delaware corporation (the “Company”, “we”, “us”, or “our”), of record as of the close of business on [—], 2026 (the “Record Date”), pursuant to Section 14© of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the rules and regulations prescribed thereunder. Only stockholders of record as of the Record Date are entitled to receive this Information Statement.
The purpose of this Information Statement is to notify our stockholders that on December [—], 2025, certain stockholders of the Company (the “Consenting Stockholders”), collectively holding approximately [—]% of the Company’s shares of common stock, par value $0.01 per share (the “Common Stock”), approved by written consent in lieu of a special meeting (the “Written Consent”), in accordance with Section 228 of the Delaware General Corporation Law (the “DGCL”) and Article [—] of the Company’s Amended and Restated Certificate of Incorporation, the following actions (the “Corporate Actions”):
1. The Preferred Stock Conversion and Issuance: the issuance of Company Common Stock upon the conversion (the “Preferred Stock Conversion”) of the Series A Convertible Preferred Stock, par value $0.01 per share of the Company (the “Preferred Stock”);
2. The Notes Conversion and Issuance: the issuance of Company Common Stock upon the conversion of the convertible notes (the “Notes Conversion”) issued by the Company pursuant to that certain Securities Purchase Agreement, entered into by the Company and certain institutional investors on December 1, 2025, and amended by that certain Amendment No. 1 to Securities Purchase Agreement on December 8, 2025 (the “SPA”);
3. The Reverse Stock Split: a 1-for-10 reverse stock split (the “Reverse Stock Split”) of the Company’s Common Stock, a change in the par value per share of the Company’s Common Stock from $0.01 to $0.001 (the “Change in Par Value”), and a corresponding amendment to the Company’s Amended and Restated Certificate of Incorporation (the “Charter”) to (i) authorize the Board of Directors to effect the Reverse Stock split and (ii) the Change in Par Value;
1
4. The 2024 Plan Share Increase: an amendment to the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan (the “2024 Plan”) to increase the number of shares of Common Stock issuable under the 2024 Plan to 2,096,679; and
5. The 2025 Equity Plan: the approval and adoption the Apimeds Pharmaceuticals US, Inc. 2025 Equity Incentive Plan (the “2025 Plan”).
The Written Consent was entered into and the Corporate Actions were approved by the Consenting Stockholders in connection with that certain Agreement and Plan of Merger (the “Merger Agreement”), attached as Annex A, which was entered into and closed on December 1, 2025, by the Company, Apimeds Merger Sub, Inc., a Delaware corporation (“Merger Sub”), MindWave Innovations Inc, a Delaware corporation (“MindWave”), Lokahi Therapeutics, Inc., a Nevada corporation (“Bio Sub”), and Erik Emerson, solely in his capacity as representative for the Bio Business (the “Bio Business Representative”). The transactions contemplated by the Merger Agreement are referred to herein as the “Transactions” and the closing of the Transactions is referred to herein as the “Closing”.
Pursuant to the terms and conditions of the Merger Agreement, immediately prior to the Closing, a certificate of merger (the “Certificate of Merger”) was filed with the Secretary of State of the State of Delaware (the “DE SOS”) (such time of the filing of the Certificate of Merger, the “Effective Time”), in accordance with the DGCL. Pursuant to the Certificate of Merger, Merger Sub was merged with and into MindWave (the “Merger”), with MindWave surviving the Merger as the surviving corporation (the “Surviving Corporation”). As a result of the Merger, MindWave became a direct wholly owned subsidiary of the Company. At the Effective Time, all of the property, rights, privileges, powers and franchises of MindWave and Merger Sub vested in the Surviving Corporation and all of the debts, liabilities and duties of MindWave and Merger Sub became the debts, liabilities and duties of the Surviving Corporation. The Closing occurred simultaneously with the execution and delivery of the Merger Agreement on the Closing Date.
At the Effective Time, (i) each share of MindWave common stock issued and outstanding immediately prior to the Effective Time shall be canceled and converted into the right to receive a portion of the Merger Consideration, consisting of shares of Company Preferred Stock; and (ii) each holder of such shares shall receive, for each share of MindWave Common Stock held immediately prior to the Effective Time, a pro rata portion of the Merger Consideration, allocated as follows: (A) a number of duly authorized, validly issued, fully paid and nonassessable shares of Company Common Stock, such that the aggregate number of shares of Company Common Stock issued to all holders of MindWave Common Stock shall equal 0% of the total number of shares of Company Common Stock issued and outstanding as of the date of the Merger Agreement (the “Common Stock Cap”), with each holder’s allocation rounded down to the nearest whole share; and (B) a number of duly authorized, validly issued, fully paid and nonassessable shares of Company Preferred Stock, such that, immediately following the Effective Time, the holders of MindWave Common Stock collectively hold, on an as-converted to Company Common Stock basis, 90.9% of the total issued and outstanding equity securities of the Company (exclusive of the Company Common Stock issued pursuant to clause (A) and calculated on a fully diluted basis). The shares of Company Common Stock and Company Preferred Stock issued to holders of MindWave Common Stock pursuant to the Merger Agreement, on an as-converted and fully diluted basis, shall collectively represent 90.9% of the equity capital of the Company as of the Closing. For purposes of the Merger Agreement, the shares of Company Common Stock, Company Preferred Stock, and MindWave Common Stock issued pursuant to the Merger Agreement are collectively referred to as the “Merger Consideration.”
Additional information regarding the Merger Agreement is contained in the Company’s Current Report on Form 8-K filed with the SEC on December 10, 2025.
The Company’s Common Stock is listed on the NYSE American LLC (“NYSE American”) and the Company is subject to NYSE American’s rules and regulations, including NYSE American Company Guide Sections 711-713, which require stockholder approval prior to an issuance of securities in connection with: (i) the acquisition of the stock or assets of another company; (ii) equity-based compensation of officers, directors, employees or consultants; and (iii) a change of control.
2
Pursuant to Section 228 of the DGCL, we are required to provide prompt notice of the taking of the corporate action described above without a meeting of stockholders to all stockholders who did not consent in writing to the Corporate Actions. This Information Statement serves as the notice required by Section 228 of the DGCL.
In accordance with the rules and regulations of the U.S. Securities and Exchange Commission, the Corporate Actions will not become effective until at least twenty (20) calendar days after we send this Information to our stockholders of record as of the Record Date (the “Action Effective Time”).
[Furthermore, notwithstanding the approval provided by the Consenting Stockholders, the Board of Directors retains sole discretion to implement or abandon the Reverse Stock Split based on its determination of whether effecting a reverse stock split is advisable and in the best interests of the Company and its stockholders.]
THIS INFORMATION STATEMENT IS FIRST BEING SENT OR GIVEN ON OR ABOUT [—], 2026 TO THE HOLDERS OF OUR COMMON STOCK AS OF [—], 2026 AND IS BEING DELIVERED TO INFORM YOU OF THE CORPORATE ACTIONS DESCRIBED HEREIN BEFORE SUCH ACTION TAKE EFFECT IN ACCORDANCE WITH RULE 14C-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED (THE “EXCHANGE ACT”).
WE ARE NOT ASKING YOU FOR A PROXY, YOU ARE REQUESTED NOT TO SEND US A PROXY AND ACCORDINGLY NO PROXY CARD HAS BEEN ENCLOSED WITH THIS INFORMATION STATEMENT. EXISTING STOCKHOLDERS WILL RETAIN THEIR EXISTING COMMON STOCK.
The entire cost of furnishing this Information Statement will be borne by the Company. We will request brokerage houses, nominees, custodians, fiduciaries and other like parties to forward this Information Statement to the beneficial owners of our voting securities held of record by them, and we will reimburse such persons for out-of-pocket expenses incurred in forwarding such material.
3
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This Information Statement and other documents referenced herein contain certain statements that constitute “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act. The words “anticipate,” “expect,” “believe,” “goal,” “plan,” “intend,” “estimate,” “may,” “will,” and similar expressions and variations thereof are intended to identify forward-looking statements but are not the exclusive means of identifying such statements. Those statements appear in this Information Statement and the documents referenced herein and include statements regarding the intent, belief or current expectations of the Company and management that are subject to known and unknown risks, uncertainties and assumptions and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed in or implied by such forward-looking statements. More information about the risks we face is described under the section titled “Risk Factors” in our Annual Report on Form 10-K for the year ending December 31, 2024 (the “Annual Report”) and any subsequently filed Quarterly Report on Form 10-Q (“Quarterly Reports”).
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely upon forward-looking statements as predictions of future events. The events and circumstances reflected in the forward-looking statements may not be achieved or occur or may not occur within the anticipated time frame and actual results could differ materially from those projected in the forward-looking statements. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events or otherwise.
4
The following questions and answers briefly address some questions that you, as Company stockholder, may have regarding the Corporate Actions. You are urged to read this Information Statement carefully and the other documents referred to in this Information Statement in their entirety because this section may not provide all the information that is important to you regarding the Corporate Actions. See “Summary” for a summary of important information regarding the Corporate Actions. Additional important information is contained in the annexes to, and the documents incorporated by reference in this Information Statement. You may obtain the information incorporated by reference in this Information Statement, without charge, by following the instructions in the section titled “Where You Can Find More Information.”
Why am I receiving this Information Statement?
You are receiving this Information Statement because the Consenting Stockholders approved, in the Written Consent, the Corporate Actions without a meeting of the stockholders of the Company. The Corporate Actions are listed below:
1. The Preferred Stock Conversion and Issuance: the issuance of Company Common Stock upon the conversion (the “Preferred Stock Conversion”) of the Series A Convertible Preferred Stock, par value $0.01 per share of the Company (the “Preferred Stock”);
2. The Notes Conversion and Issuance: the issuance of Company Common Stock upon the conversion of the convertible notes (the “Notes Conversion”) issued by the Company pursuant to that certain Securities Purchase Agreement, entered into by the Company and certain institutional investors on December 1, 2025, and amended by that certain Amendment No. 1 to Securities Purchase Agreement on December 8, 2025 (the “SPA”);
3. The Reverse Stock Split: a 1-for-10 reverse stock split (the “Reverse Stock Split”) of the Company’s Common Stock, a change in the par value per share of the Company’s Common Stock from $0.01 to $0.001 (the “Change in Par Value”), and a corresponding amendment to the Company’s Amended and Restated Certificate of Incorporation (the “Charter”) to (i) authorize the Board of Directors to effect the Reverse Stock split and (ii) the Change in Par Value;
4. The 2024 Plan Share Increase: an amendment to the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan (the “2024 Plan”) to increase the number of shares of Common Stock issuable under the 2024 Plan to 2,096,679; and
5. The 2025 Equity Plan: the approval and adoption the Apimeds Pharmaceuticals US, Inc. 2025 Equity Incentive Plan (the “2025 Plan”).
This Information Statement is being furnished to inform the Company’s stockholders of the Corporate Actions, which do not require any further stockholder approval.
What is the record date for determining stockholders entitled to receive this Information Statement?
The record date for determining the stockholders entitled to receive this Information Statement is [—], 2026 (the “Record Date”). Only stockholders of record at the close of business on the Record Date are entitled to receive this Information Statement.
Did stockholders vote on these matters?
No meeting or vote of stockholders was held. The Corporate Actions described in this Information Statement were approved by the Written Consent of the Consenting Stockholders, in accordance with Section 228 of the DGCL.
When will these actions become effective?
In accordance with Rule 14c-2(b) under the Exchange Act, the Corporate Actions will become effective no earlier than twenty (20) calendar days after this Information Statement is sent or mailed to the stockholders of record of the Company as of the Record Date.
5
Is any stockholder action required by me?
No. You are not required to take any actions as a result of this Information Statement.
Where can I find more information about the Company?
The Company files period reports, proxy statements, and other information with the SEC. This information is available on the website maintained by the SEC at www.sec.gov. For a more detailed description of the available information, please refer to “Where You Can Find More Information.”
6
BACKGROUND OF THE MERGER AGREEMENT
The Written Consent was entered into and the Corporate Actions were approved by the Consenting Stockholders in connection with that certain Agreement and Plan of Merger (the “Merger Agreement”), attached as Annex A, which was entered into and closed on December 1, 2025, by the Company, Apimeds Merger Sub, Inc., a Delaware corporation (“Merger Sub”), MindWave Innovations Inc, a Delaware corporation (“MindWave”), Lokahi Therapeutics, Inc., a Nevada corporation (“Bio Sub”), and Erik Emerson, solely in his capacity as representative for the Bio Business (the “Bio Business Representative”). The transactions contemplated by the Merger Agreement are referred to herein as the “Transactions” and the closing of the Transactions is referred to herein as the “Closing”.
Merger Agreement
Pursuant to the terms and conditions of the Merger Agreement, immediately prior to the Closing, a certificate of merger (the “Certificate of Merger”) was filed with the Secretary of State of the State of Delaware (the “DE SOS”) (such time of the filing of the Certificate of Merger, the “Effective Time”), in accordance with the DGCL. Pursuant to the Certificate of Merger, Merger Sub was merged with and into MindWave (the “Merger”), with MindWave surviving the Merger as the surviving corporation (the “Surviving Corporation”). As a result of the Merger, MindWave became a direct wholly owned subsidiary of the Company. At the Effective Time, all of the property, rights, privileges, powers and franchises of MindWave and Merger Sub vested in the Surviving Corporation and all of the debts, liabilities and duties of MindWave and Merger Sub became the debts, liabilities and duties of the Surviving Corporation. The Closing occurred simultaneously with the execution and delivery of the Merger Agreement on the Closing Date.
Transaction Consideration
At the Effective Time, (i) each share of MindWave common stock issued and outstanding immediately prior to the Effective Time shall be canceled and converted into the right to receive a portion of the Merger Consideration, consisting of shares of Company Preferred Stock; and (ii) each holder of such shares shall receive, for each share of MindWave Common Stock held immediately prior to the Effective Time, a pro rata portion of the Merger Consideration, allocated as follows: (A) a number of duly authorized, validly issued, fully paid and nonassessable shares of Company Common Stock, such that the aggregate number of shares of Company Common Stock issued to all holders of MindWave Common Stock shall equal 0% of the total number of shares of Company Common Stock issued and outstanding as of the date of the Merger Agreement (the “Common Stock Cap”), with each holder’s allocation rounded down to the nearest whole share; and (B) a number of duly authorized, validly issued, fully paid and nonassessable shares of Company Preferred Stock, such that, immediately following the Effective Time, the holders of MindWave Common Stock collectively hold, on an as-converted to Company Common Stock basis, 90.9% of the total issued and outstanding equity securities of the Company (exclusive of the Company Common Stock issued pursuant to clause (A) and calculated on a fully diluted basis). The shares of Company Common Stock and Company Preferred Stock issued to holders of MindWave Common Stock pursuant to the Merger Agreement, on an as-converted and fully diluted basis, shall collectively represent 90.9% of the equity capital of the Company as of the Closing. For purposes of the Merger Agreement, the shares of Company Common Stock, Company Preferred Stock, and MindWave Common Stock issued pursuant to the Merger Agreement are collectively referred to as the “Merger Consideration.”
Additional information regarding the Merger Agreement is contained in the Company’s Current Report on Form 8-K filed with the SEC on December 10, 2025.
7
BACKGROUND OF THE CONVERTIBLE NOTE FINANCING
On December 1, 2025, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”), attached as Annex B, with an institutional investor (the “Investor”), pursuant to which the Company agreed to issue senior convertible notes (the “Notes”) in an aggregate principal amount of up to $120,900,000 at an 8% original issue discount (the “Note Financing”), over a 24-month period (the “Commitment Period”).
The Notes bear no interest unless an event of default occurs. The Notes constitute senior unsecured obligations of the Company. Pursuant to the terms of the Registration Rights Agreement, entered into by the Company and the Investor in connection with the Securities Purchase Agreement on December 8, 2025, (the “Registration Rights Agreement”), the Company is required to file a resale registration statement on Form S-1 (the “Resale Registration Statement”) within 45 days of closing and have it declared effective within 75 days (or 120 days in the event of a full SEC review), subject to certain extensions, registering the shares of Company Common Stock issuable pursuant to the terms of the Notes. Delays beyond these deadlines will result in liquidated damages of 1% of the Redemption Value (as defined in the Registration Rights Agreement) for each 30-day period.
In connection with the Closing, $10,875,000 of the principal amount of the Notes shall be made available to the Company, and an additional $2,175,000 shall be funded upon the effectiveness of the Resale Registration Statement. The Investor shall have the right, at its sole discretion, to purchase up to an aggregate of $13,075,000 of such additional Notes in one or more closings. The Investor shall have the ability to purchase, subject to mutual consent of the parties, any amount remaining under the Notes. The Notes shall mature on the 12-month anniversary of their issuance at 100% of the face value. The Investor has the right to convert the notes at 80% of the lowest daily volume weighted average price during the 5-trading day period ending and including the date of conversion (the “Conversion Price”). Monthly conversions using the Conversion Price shall be limited to the greater of (i) 20% of the aggregate of the daily traded value during such calendar month period, and (ii) $2.25 million.
The Notes include customary negative and affirmative covenants for transactions of this type. The Investor has agreed to a no net short provision, subject to certain carve-outs. The Investor also has the right to participate in up to 30% of any subsequent financing until the later of six months after the Commitment Period or the Notes’ maturity date. The Company agreed to obtain voting agreements from stockholders representing at least 51% of all outstanding shares before Closing.
Under the Securities Purchase Agreement, the Company is obligated to seek stockholder approval for the Note Conversion. To facilitate this approval, the Company entered into voting agreements (each, a “Voting Agreement”) with certain officers, directors, and stockholders, pursuant to which these parties agreed to vote all shares of Company Common Stock they hold in favor of the Note Conversion.
On December 8, 2025, the Company and the Investor entered into Amendment No. 1 to the Securities Purchase Agreement, attached as Annex C, under which the parties (i) clarified how long the Company is prohibited from entering into a variable rate transaction, (ii) expanded the notification rights of the Investor if another funding event occurs, and (iii) extended the date for the Company to complete the Initial Closing (as such term is defined in the Securities Purchase Agreement).
In connection with the Securities Purchase Agreement, on January 8, 2025, the Company entered into that certain Amendment and Exchange Agreement, attached as Annex D, which amends certain terms of the Securities Purchase Agreement, by authorizing a new series of senior secured convertible notes in the aggregate original principal amount of $120,900,000, in exchange of the Notes.
Additional information on the Securities Purchase Agreement is contained in the Company’s Current Reports on Form 8-K filed with the SEC on December 2, 2025 and December 10, 2025.
8
Apimeds Pharmaceuticals US, Inc.
Apimeds Pharmaceuticals US, Inc. (NYSE American: APUS) (the “Company”) is a clinical-stage biopharmaceutical company focused on developing non-opioid, biologic-based therapies for pain management.
The Company was formed as a corporation in May 2020 and was incorporated in the State of Delaware. On August 21, 2021, Apimeds, Inc., a stockholder of the Company (“Apimeds Korea”), and the Company entered into a business agreement, under which the Company was designated to operate a pharmaceutical business which provides the biological drug named Apitox™ to clients in the biological drug commercial transaction area.
The Company is in the process of developing Apitox™, a proprietary intradermally administered bee venom-based toxin which completed a positive Phase 3 trial for the treatment of pain associated with Osteoarthritis (“OA”) in 2018 and is now proceeding with FDA discussions on next steps in approval. In the future, the Company plans to investigate potential uses for Apitox™ for in treating multiple sclerosis (“MS”) and intends to conduct non-registered corporate sponsorship studies to identify appropriate MS patient populations. Apitox™ is currently marketed and sold by Apimeds Korea in South Korea (Republic of Korea) as “Apitoxin” for the treatment of osteoarthritis.
The success of the Company is dependent on obtaining the necessary regulatory approvals of its product candidates, marketing its products and achieving profitable operations. The continuation of the research and development activities and the commercialization of its products, if approved, are dependent on the Company’s ability to successfully complete these activities and to obtain additional financing through a combination of financing activities and operations. It is not possible to predict either the outcome of future research and development or commercialization programs, or the Company’s ability to fund these programs.
MindWave Innovations Inc
MindWave Innovations Inc (“MindWave”) is a leading provider of institutional Digital Asset Treasury (DAT)
solutions, specializing in compliant Bitcoin treasury infrastructure, AI-driven yield capabilities, ClimateTech impact systems, and AdTech engagement platforms. Its multi-vertical ecosystem is powered by its native token, $NILA, which enables governance, utility, and value flow across its blockchain-integrated operations.
MindWave was formed as a corporation on November 10, 2025 and was incorporated in the State of Delaware.
9
INFORMATION ABOUT THE BUSINESS OF APIMEDS PHARMACEUTICALS US, INC.
Unless the context otherwise indicates or requires, all references in this section to “we,” “us,” “our,” “our company” “the Company” and “Apimeds US” refer to Apimeds Pharmaceuticals US, Inc.
We are a clinical stage biopharmaceutical company in the process of developing Apitox, an intradermally administered bee venom-based toxin. Our focus is primarily on developing innovative therapies that address inflammation and pain management symptoms associated with knee OA and, to a lesser extent, MS. Apitox is currently marketed and sold by Apimeds Inc. (“Apimeds Korea”) in South Korea as “Apitoxin” for the treatment of OA. Apimeds US is not associated with the market, sale and revenues generated from Apitoxin in South Korea, and Apitoxin has not yet been approved by the FDA for any indication.
Apitox is a purified, pharmaceutical grade venom (bee venom), of the Apis mellifera, or western honeybee, which is classified by the FDA as an active pharmaceutical ingredient (“API”). Bee venom has been used in Asia and Europe to treat pain for hundreds of years. While not FDA approved in a controlled, prescription based biologic environment for defined indications, the use of bee venom has been FDA approved as a “under the skin injection” to reduce the allergic reactions to bee stings. Apimeds Korea has developed a proprietary method and process for turning extracted bee venom into a lyophilized powder for reconstitution prior to intradermal dose injections, which they sell in South Korea as Apitoxin. We intend to use a similar process with respect to Apitox, pursuant to the Business Agreement, which gives us a license to utilize all prior clinical development data associated with Apitoxin. The advancement of extracted bee venom for treatment of inflammatory conditions, including but not limited to knee OA and MS is speculative but based on direction provided by prior clinical data.
Apimeds Korea successfully completed Phase I, Phase II, and Phase III trials in OA in 2003, at which point Apitoxin was approved by the Korean Ministry of Food and Drug Safety (“MFDA”) to treat pain and mobility in patients with OA. Since 2003, a post-marketing/approval safety study in South Korea followed 3,194 patients from 2003 through 2009, with no serious adverse events. The purpose of a Phase I trial is to test to determine whether a new treatment is safe and look for the best way to give the treatment. Phase II trials test to determine whether a condition or disease responds to the new treatment. Phase III trials test to determine whether a new treatment is better than a standard treatment.
In 2013, the first of two required U.S. Phase III clinical trials was authorized to enroll patients to study the use of Apitoxin to study the same indication as approved in South Korea in 2023 — treatment of pain and lack of mobility in patients with OA (the “Apimeds Korea Phase III OA Trial”). The Apimeds Korea Phase III OA Trial (330 patients) was completed in 2018, and displayed no serious adverse events.
Based on the results from the Apimeds Korea Phase III OA Trial, which demonstrated therapeutic (statistical and clinically significant improvements in all outcome measures of pain, physical function, and disease assessment) effect compared to the placebo group, but in combination with prior development by Apimeds Korea, did not meet the FDA’s standards for approval, as the study population was too small and the methods for handling missing data were inadequate, resulting in a study that did not demonstrate a significant treatment effect. We will be pursuing a second Phase III trial to meet agreed upon FDA standards. Based on results from the Apimeds Korea Phase III OA Trial, we have evaluated the most appropriate population, defined as advanced knee OA patients, which will range from defined grade 2, 3 and 4 within this treatment group, to continue to progress our own Phase III trial. Pursuant to our previous correspondence with the FDA, we have designed and will implement our Phase III trial to best address our patient population, appropriate dosing, and the most effective way to evaluate Apitox in meeting the patient population’s needs.
We believe the progress we are making in clinical trials provides us support in our belief in the potential of Apitox to be an innovative therapy. We aim to treat the inflammation and pain management symptoms associated with knee OA and to help manage the devastating symptoms of this disease. In the future, we also aim to leverage our research in knee OA to investigate how Apitox may be used to treat similar symptoms associated with MS.
Treatment of OA
OA is typically treated with painkillers known as non-steroidal anti-inflammatory drugs (NSAIDs). These medications have an anti-inflammatory and pain-relieving effect. These medications include ibuprofen (Motrin, Advil) naproxen (Aleve) and diclofenac (Voltaren and others). All of these medications work by blocking enzymes that cause pain and
10
swelling. The problem is that some of those enzymes also help blood to clot and protect the lining of your stomach. Without them, you can bruise easily, develop ulcers and may even bleed in your intestines. NSAIDs also increase your chance of heart attack, stroke and heart failure. The risk increases the longer you use them and the more you take. We believe Apitox could be a successful alternative to NSAIDs in the treatment of the inflammation and pain management symptoms associated with OA without the harmful side effects.
According to MedicalNewsToday, OA is the most common form of arthritis, affecting around 500 million people worldwide, or around 7% of the global population. Currently, in the United States, over 32 million people suffer from OA. As the 15th highest cause of years lived with disability (YLDs) worldwide, the burden OA poses to individuals is substantial, characterized by pain, activity limitations, and reduced quality of life. The economic impact of OA, which includes direct and indirect (time) costs, is also substantial, ranging from 1 to 2.5% of gross national product (GNP) in countries with established market economies, like the United States. Though trends in OA prevalence vary by geography, the prevalence of OA is projected to rise in regions with established market economies such as North America and Europe, where populations are aging and the prevalence of obesity is rising.
While OA can occur in any joint, it occurs most frequently in the knee, which, according to ScienceDirect, currently accounts for 365 million cases worldwide and 61% of YLDs lost due to OA, followed by the hand.
Our current efforts are focused on the development of Apitox in the United States for the treatment of inflammation and pain management relating to OA in the knee.
Treatment of MS
Additionally, we believe the previous clinical trial success of Apimeds Korea with respect to the use of Apitoxin to treat symptoms associated with knee OA, and pending the success of our anticipated Phase III trial in knee OA, we will be in a position to further explore the use of Apitox as a potential treatment for the symptoms of MS. MS is a chronic disease of the central nervous system. It is an autoimmune condition that is characterized by the body’s own immune cells (macrophages and lymphocytes) attacking the myelin that coats nerve cells, which can lead to inflammation throughout the central nervous system. MS is an unpredictable disease that affects people differently. Some people with MS may have only mild symptoms. Others may lose their ability to see clearly, write, speak, or walk when communication between the brain and other parts of the body becomes disrupted.
MS is the most common progressive neurologic disease of young adults worldwide. A study funded by the National MS Society estimates that nearly one million individuals are currently affected by this disease in the United States. The total economic burden of MS in the United States is estimated to be $85.4 billion, with $63.3 billion in direct medical costs and $22.1 billion in indirect and nonmedical costs. MS typically affects patients at a young age, resulting in a greater loss of productivity and quality of life.
Beta interferon drugs are among the most common medications used to treat MS. Interferons are signaling molecules that regulate immune cells. Potential side effects of these drugs include flu-like symptoms (which usually fade with continued therapy), depression, or elevation of liver enzymes.
Pain from MS can be felt in different parts of the body. Trigeminal neuralgia (facial pain) is treated with anticonvulsant or antispasmodic drugs, or less commonly, painkillers. Central pain, a syndrome caused by damage to the brain and/or spinal cord, can be treated with gabapentin and nortriptyline. Treatments for chronic back or other musculoskeletal pain may include heat, massage, ultrasound, and physical therapy.
OA and the Current Standard of Care
OA is a degenerative joint disease in which the tissues in the joint break down over time. It is the most common type of arthritis and is more common in older people. People with osteoarthritis usually have joint pain and, after rest or inactivity, stiffness for a short period of time.
There are four stages of OA: (1) Minor — minor wear-and-tear in the joints and little to no pain in the affected area, (2) Mild — more noticeable bone spurs, the affected area feels stiff after sedentary periods and patients may need a brace, (3) Moderate — cartilage in the affected area begins to erode, the joint becomes inflamed and causes discomfort during normal activities, and (4) Severe — the patient is in a lot of pain, the cartilage is almost completely gone leading to an inflammatory response from the joint, and overgrowth of bony spurs may cause severe pain.
11
With the progression of OA of the knee, there is obvious joint inflammation which causes frequent pain when walking, running, squatting, extending or kneeling. Along with joint stiffness after sitting for long or when waking up in the morning, there may be popping or snapping sounds when walking.
The data from the Apimeds Korea Phase III OA Trial suggest that Apitox would have the most potential in treating OA in stages 3 and 4.
MS and the Current Standard of Care
MS is increasingly recognized as a neurodegenerative disease triggered by an inflammatory attack of the central nervous system. There is no cure for multiple sclerosis. Treatment typically focuses on speeding recovery from attacks, reducing new radiographic and clinical relapses, slowing the progression of the disease, and managing MS symptoms.
MS is unpredictable and can vary substantially from person to person. MS is divided into four types: clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS), secondary progressive MS (SPMS) and primary progressive MS (PPMS).
CIS refers to a first episode of neurologic symptoms caused by inflammation and demyelination in the central nervous system.
RRMS, the most common disease course, shows clearly defined attacks of new or increasing neurologic symptoms. These attacks are also called relapses or exacerbations. They are followed by periods of partial or complete recovery, or remission. In remissions, all symptoms may disappear or some symptoms may continue and become permanent. However, during those periods, the disease does not seem to progress.
SPMS follows the initial relapsing-remitting course. Some people diagnosed with RRMS eventually go on to have a secondary progressive course, in which neurologic function worsens progressively or disability accumulates over time.
With PPMS, neurologic function worsens or disability accumulates as soon as symptoms appear, without early relapses or remissions. PPMS can be further characterized as either active (with an occasional relapse and/or evidence of new MRI activity over a specified period of time) or not active, as well as with progression (evidence of disability accrual over time, with or without relapse or new MRI activity) or without progression.
Patients with MS tend to be more educated about their disease and better organized than patients with other diseases, resulting in patients that are aggressive in their approach to treatment. This is due to MS impacting otherwise healthy people in the prime of their lives.
MS treatment has undergone significant evolution in the last ten years with the development and approval of certain new drugs, including several oral agents such as Ocrevus, in the United States. These new agents not only give patients additional treatment options, but also have improved the efficacy and safety of treatment for MS overall. In general, these drugs are “disease modifying agents,” intended to slow down the immune mediated damage to the myelin sheaths that underlie symptoms in MS. However, they often do not adequately address the symptoms that MS patients experience such as walking problems, bladder control, dizziness, and especially pain. A 2022 study estimated that the average cost of treatment for patients with MS is approximately $88,000 annually. The out-of-pocket expense for patients can be significantly reduced through certain insurance plans. However, we believe there is the ability for Apitox to be positioned as an important and cost-effective therapy.
We believe the data from the Apimeds Korea Phase III OA Trial suggest that Apitox may have the potential as an adjunctive therapy for all four types of MS. We intend to Apitox as a potential adjunctive therapy through non-registered corporate sponsorship studies to begin determining the appropriate MS patient populations.
Market Opportunity
We believe there is a significant market opportunity in the United States for Apitox in the treatment of certain symptoms of knee OA and eventually MS. According to Precedence Research the osteoarthritis therapeutics market size accounted for $8.28 billion in 2022 and it is expected to hit around $20.24 billion by 2032, expanding at a CAGR of 9.4% from 2023 to 2032. Although OA can damage any joint, the disorder most commonly affects joints in your hands, knees, hips and spine. OA symptoms can usually be managed, although the damage to joints can’t be reversed. Apitox has certain anti-inflammatory properties, which we believe give it significant potential to help treat the symptoms of certain chronic diseases that involve difficult to control pain and inflammation.
12
According to Pharmaceutical Technology the MS market size in the United States accounted for $10.73 billion in 2022 and is expected to hit $24.4 billion by 2030, expanding at a CAGR of 10.32%. Starting in the first quarter of 2025, we intend to begin the early prosecution of appropriate MS patient populations through non-registered corporate sponsorship studies. Subject to FDA approval, our development of Apitox in the United States will in the near term, have two distinct focuses (i) the treatment of the certain symptoms of knee OA and (ii) the quality of life issues surrounding knee OA, such as pain and lack of mobility.
Living with a chronic disease is challenging, as it interferes with physical, mental, and social functions and thus greatly affects a person’s quality of life. Indeed, chronically ill patients are facing major struggles such as higher expenditures, social isolation and loneliness, disabilities, fatigue, pain/discomfort, feelings of distress, anger, hopelessness, frustration, anxiety, and depression. There is the general assumption that symptom reduction increases a patient’s quality of life. Our approach with Apitox centers around this concept — effectively treating certain symptoms of the patient’s disease, thus improving their overall quality of life. Bee venom has been shown to have anti-inflammatory effects. At low doses, bee venom can suppress inflammatory cytokines such as interleukin-6 (IL-6), IL-8, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α). A decrease in the signaling pathways responsible for the activation of inflammatory cytokines, such as nuclear factor-kappa B (NF-κB), extracellular signal-regulated kinases (ERK1/2) and protein kinase Akt, and porphyromonas gingivalis lipopolysaccharide (PgLPS)-treated human keratinocytes has been associated with treatments involving bee venom. We believe the driver of pain in the highest category of OA is correlated to the key inflammatory elements treated by bee venom, meaning the evaluation of our Phase III data may lead to a small indication for narcotic use reduction in the treatment of stage 4 OA.
Our Product Candidate
Apitox is purified honeybee (Apis mellifera) venom manufactured as a lyophilized powder for reconstitution in 0.5% preservative-free lidocaine (lmg/mg) prior to intradermal dose injections that are administered up to 1,500 micrograms per weekly visit. The biologically active components include melittin (40-50%), apamin (2-3%), mast cell degranulating (“MCD”) peptide (Peptide 401,2-3%), phospholipase A2 (10-15%), hyaluronidase (1.5-2%) and other components in small amounts, including dopamine and norepinephrine. According to a publication entitled “Pharmacological effects and mechanisms of bee venom and its main components: Recent progress and perspective” by Shi et al., certain components of honeybee venom have been found to have both anti-inflammatory and analgesic effects. The anti-inflammatory and analgesic effects are attributed to the presence of Peptide 401, adolapin and other components that inhibit prostaglandin synthesis. The hormone-stimulating effects are attributed to the presence of melittin, cardiopep and other components that stimulate the pituitary-adrenal axis to produce cortisol. Results from an animal study entitled “Effect of bee venom and melittin on plasma cortisol in the unanesthetized monkey” published by Vick et al., indicate that melittin appears to stimulate the production of cortisol from the adrenal gland. The immune-modulating effects, especially as it pertains to MS, are suggested to be mediated by CD4+CD2S+Foxp3+ regulatory T cells (Tregs) that are influenced by phospholipase A2. While the exact mechanism of action of Apitox is not fully understood, research such as the publication entitled “Therapeutic Use of Bee Venom and Potential Applications in Veterinary Medicine” by Bava et al., suggests that certain components in Apitox may ameliorate immune-inflammatory responses associated with MS. Such studies suggested that treatments with melittin prevent inflammatory cytokine expression and produces anti-inflammatory effects. The proposed indication for Apitox is to provide add-on therapy for the signs and symptoms of MS in patients whose condition is relapsing-remitting (RRMS), primary-progressive (PPMS) or secondary progressive (SPMS).
Clinical Development History
Founded in 1989, Apimeds Korea pursued a traditional drug development process in South Korea for Apis mellifera, the bee venom API for Apitoxin. Apimeds Korea completed a formal preclinical study to validate dosing and safety for human administration with a focus on antigenicity and toxicology in 1993.
A Phase I trial was completed in 1994, studying the toxicity and safety of Apitoxin in 20 healthy subjects. The purpose of the Phase I trial was to determine if therapeutic doses of Apitoxin was safe and to identify possible side-effects, if any. Injections of Apitoxin were given two to three times a week, for a total of 12 sessions spanning over four to six weeks. Laboratory and physical examination of the subjects included (i) serum cortisol levels (to see if Apitoxin stimulated the release of cortisol), (ii) serum ionized calcium level (to determine if Apitoxin decreased the serum calcium level), (iii) urinalysis, (iv) hematology and blood chemistry, and (v) vital signs. The Phase I trial demonstrated that there were no significant changes pre- and post-testing of the serum cortisol levels, serum ionized calcium levels,
13
hematology, blood chemistry, urinalysis, and vital signs after the subjects were injected with Apitoxin according to the protocol. There were no significant physiological changes in the clinical evaluations of the subjects and localized itching was the most frequent side effect and was managed with ice packs or external anti-itching gels. No severe side effects or aftereffects were observed. The Phase I trial indicated that Apitox is safe for humans when applied in therapeutic doses.
The Phase I trial was followed by a Phase II trial in 101 subjects to determine the efficacy of Apitoxin at various dose levels. This was a randomized active-controlled clinical trial with three groups receiving the study drug at various dose levels and one group receiving the control drug (nabumetone) for a six-week period. Patients received twice weekly injections of Apitox intradermally at dosages titrated to a maximum of 0.7 mg (Group A), 1.5 mg (Group B), and 2.0 mg (Group C) for a period of six weeks. Control group patients (Group D) received 1,000 mg of nabumetone orally each day for the same six-week period. There were 25, 26, 25 and 25 patients assigned to Groups A, B, C and D, respectively. Efficacy of treatment was evaluated by the physician investigators using a 4-point Likert-like symptom severity rating scale developed by the authors to assess Pain, Disability and Physical Signs. A similar 5-point scale was used for patient self-evaluation. Safety of the Apitoxin injection was evaluated by patient reaction, hematologic examination, and laboratory chemistry analysis of blood and urine. Efficacy data was reported for the 81 patients who completed the study. While there were no significant differences in symptom severity scores among the four groups at baseline, symptom scores were significantly better in the bee venom injection groups than in the control group at six weeks and 10 weeks after the start of treatment (p<0.01). A treatment was considered effective if there was a 20% improvement from baseline in symptom scores after 6 weeks of treatment. Based on this definition, therapy demonstrated overall efficacy in 70.0% of patients in Group A, 85.7% in Group B, 90.0% in Group C, and 61.9% in Group D (drug control). Overall efficacy was significantly greater in treatment Groups B and C combined than in the nabumetone-treated control group D (p<0.0177). Importantly, efficacy of treatment among all patients treated with Apitoxin injection was greater than among nabumetone-treated patients for each category assessed: Pain: 85.2% versus 76.2%; Disability: 77.0% versus 71.4%; and Physical Signs: 62.3% vs. 23.8%. It is also noteworthy that, unlike the drug control group, the Apitoxin injection groups continued to demonstrate improved symptom scores at four weeks after the last treatment (10 weeks). There were no significant changes in vital signs or results of laboratory examinations of any patient in this clinical trial. Localized itching was experienced by all patients who received Apitox injections. Itching at the injection site generally lasted for two to three weeks; several patients had this reaction for a longer period. This Phase II study showed that Apitoxin was significantly more effective than the control drug, nabumetone, in the treatment of knee and spinal osteoarthritis patients. It clearly showed that improvement in pain, disability and physical signs was greater in the bee venom injection groups than in the nabumetone control group. No significant side effects developed at the therapeutic doses studied. However, research should be continued to minimize itching and pain at bee venom injection sites, and possible allergic reaction should always be considered with treatment at high doses.
In 2002, a formal Phase III double-blind, placebo-controlled trial was completed with 407 subjects (311 of which obeyed the trial protocol and completed the clinical study). The purpose of the Phase III trial was conducted to verify the efficacy and safety of the medicine resulting from the prior Phase I and Phase II trials. The therapeutic course treatment included a total of 12 injections over a period of 6 weeks. Final evaluations were completed in the 8th week, following two weeks of no injections. During the trial period, laboratory tests were carried out three times (before injection, in the second week, in the sixth week), and the efficacy evaluation was performed four times (before injection, in the second week, in the sixth week, and in the eighth week). Safety of the Apitoxin injection was evaluated by, hematologic examination, measurement of cortisol and calcium levels, and laboratory chemistry analysis of blood and urine. The primary efficacy variable for the trial was the ratio of the subjects who showed more than 20% improvement in the total points of test items for efficacy evaluation 6 weeks after injection, compared with the total points before injection of the medicine (the “improvement rate”). Data obtained from subjects of the clinical test were analyzed by two methods, ITT (Intention to Treat) analysis and PP (Per Protocol) Among 310 subjects who participated in the efficacy evaluation, 153 and 157 patients belonged to the Apitoxin group and the nabumetone group, respectively. For the Apitoxin group, the ratio of the subjects who showed more than 20% improvement in the total points was 48.70% (75/154 subjects, 95% confidence interval (“CI”): 40.8~56.6%), while for the nabumetone group, it was 46.15% (72/156 subjects, 95% CI: 38.3~54.0%), indicating that the improvement rate in the Apitoxin group was greater than in the nabumetone group; however, there was no statistical significance. (p=0.6533). Among a total of 407 subjects (Apitoxin group: 204; Nabumetone group: 203), 38.24% (78/204) of the Apitoxin group showed more than 20% improvement during the 6th week of injection, while 38.42% of the Nabumetone group improved by more than 20%, indicating that the two groups showed similar improvement rate (p=0.9688). The second efficacy variable was the
14
improvement rate during the 8th week (2 weeks after the completion of the final injection). According to results from comparing the total points of efficacy evaluation items during the second week after completion of injection (during the 8th week after injection) with the total points before injection, 58.44% (90/154) of the Apitoxin group showed a higher improvement rate than during the 6th week (48.70%), while 42.95% (67/156) of the Nabumetone group showed lower improvement rate than during the 6th week (46.15%). There was statistical difference in total point of efficacy evaluation items between the two groups (p=0.0064). These results suggest that even after treatment stops, the efficacy of Apitoxin continues. With respect to safety, among a total of 407 subjects who participated in the safety evaluation, 69 (33.82%) of the Apitoxin group showed an adverse event, while 59 (29.06%) of the Nabumetone indicated adverse event. These results indicate that the Apitoxin group had an elevated adverse event rate than the Nabumetone group, but there was no statistically significant difference between the two groups (p=0.3526).
In May 2003, MFDA granted approval for the use of Apitoxin in the treatment of pain and mobility in patients with OA. A post-marketing/approval safety study in South Korea followed 3,194 patients from 2003 through 2009, with no serious adverse events or negative safety signals.
In 2013, preliminary Phase III clinical trials were authorized to enroll patients by the FDA to study the same indication approved in South Korea — treatment of pain and lack of mobility in patients with OA. The results of the preliminary Phase III clinical trial indicated statistical and clinically significant improvements in all outcome measures of pain, physical function, and disease assessment in the study group. The study group included 330 patients with diagnosed osteoarthritis of the knee. The subjects were evaluated for relief of pain using Western Ontario and McMaster Osteoarthritis Index (WOMAC) and physician and patient global assessments. The primary efficacy measure was relief of pain and inflammation over a 12-week treatment period after randomization into the trial. The secondary efficacy measure was improvement of mobility. Treatment effect will be compared in a 2-1 Apitox vs active control. Compared with the placebo group (histamine), subjects in the Apitox group who received a maximum dose (1500 micrograms) at each weekly visit over 12 weeks showed a significantly more improvement in all outcome measures (WOMAC pain, WOMAC physical function, visual analog scale (“VAS”) pain, patient and physician global assessments of OA). Further, post hoc analyses showed that a statistically significant greater percentage of Apitox-treated subjects had at least a 40% and 60% reduction in WOMAC pain as compared to placebo-treated subjects. Sensitivity analyses confirmed the validity of the statistical methods and population definitions. The improvements in pain endpoints were highly significant for both the modified intention to treat and per protocol populations and the improvement was sustained during the four weeks following Apitox treatment.
Except for an expected higher incidence of injection site reactions (<5%) in the Apitox group, the overall safety profiles were comparable between the treatment groups. A serious adverse event of the anaphylactic reaction occurred in an Apitox-treated subject because of a quick injection rate. However, the subject was treated, and the event was resolved within one day. The incidence of adverse events overall was similar between the Apitox and Placebo groups (49.0% and 46.3%, respectively), and there were no clinically meaningful changes, within and between groups, in laboratory parameters, vital signs, physical examination, or electrocardiogram results.
During Apimeds Korea meetings with the FDA, the FDA highlighted concerns regarding the opioid crisis. As Apitoxin has been previously approved in South Korea, we believe Apitox could be a viable treatment option within the United States after additional clinical investigation, including our anticipated Phase III trial. Initially, Apimeds Korea elected not to pursue the OA indication in the United States based on its evaluation of potential market adoption and the existing competitive environment for OA. Based on results from the Apimeds Korea Phase III OA Trial and correspondence with the FDA, we believe we are now in a position to continue to advance our Phase III trial for knee OA.
We intend to conduct an additional Phase III trial in knee OA. Based on our previous correspondence with the FDA, we have started to design and will implement our Phase III trial to best address our patient population of patients with grade 2, 3 and 4 knee OA, appropriate dosing, and the most effective way to evaluate Apitox in meeting a patient’s needs. This trial will be an update to the plan of execution based on review of data, discussions with former principal investigators from Apimeds Korea. Upon successful completion and FDA clearance of our Phase III trial in knee OA, we will be positioned to submit a BLA.
We intend that the purpose of this trial will be to evaluate the effectiveness of Apitox in the treatment of grade 2, 3 and 4 OA of the knee. The trial will be designed with a specific focus on the identified subgroup from which we see the highest degree of benefit.
15
The following table summarizes the preliminary clinical trial activity by Apimeds Korea with respect to Apitoxin:
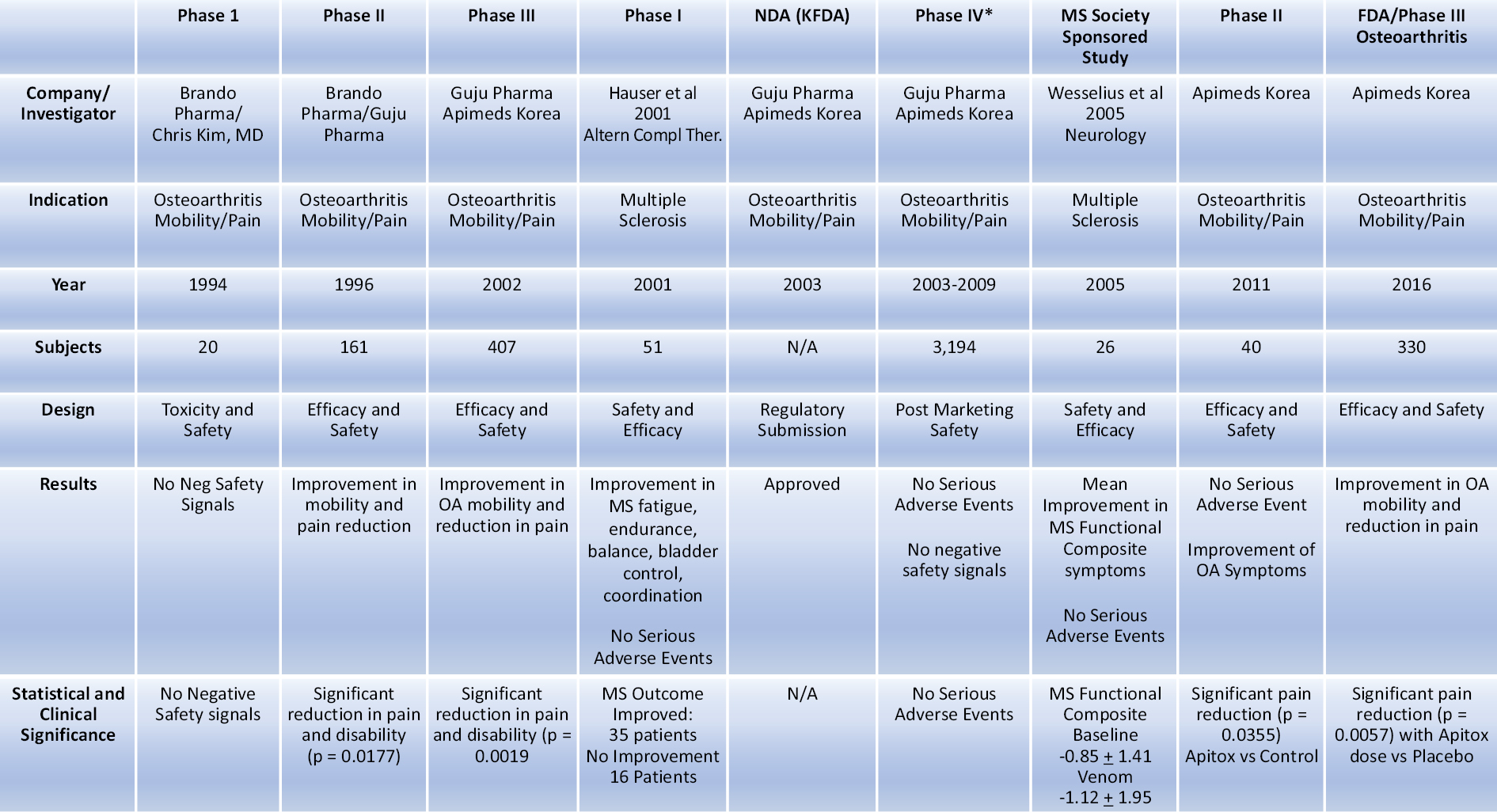
Preliminary Clinical Data in MS Patients
The United States data from the literature on bee venom studies in MS patients, Table A (Hauser et al. 2001) below, showed clinically significant improvements in disability symptoms following treatment.
In Table A, results were categorized into the following groups: dramatic disability improvement (>12 points on the Related Observable Symptom Scale (“ROSS”), good improvement (7-12 points on ROSS), minimal improvement (<7 points on ROSS), no improvement (<2 points on ROSS), and negative (any total negative response on ROSS). Descriptive analysis of the ROSS clinical outcomes showed that more than 68% of MS patients showed some kind of positive improvement in disability (dramatic, good or minimal) and 58% demonstrated a marked improvement (dramatic or good).
Table A. Summary of Patient Disability Improvement to Bee Venom Treatment Using ROSS
|
N |
% of |
Follow-up Survey |
Related Observable |
||||||
|
Dramatic |
15 |
29.4 |
% |
>30%, or |
>12 points |
||||
|
Good |
15 |
29.4 |
% |
10 – 29%, or |
7 – 12 points |
||||
|
Minimal |
5 |
9.8 |
% |
<10%, or |
<7 points |
||||
|
None |
15 |
29.4 |
% |
<2%, or |
<2 points |
||||
|
Negative |
1 |
2.0 |
% |
Any total negative response |
Any total negative response |
||||
After 1 year of bee-venom injections, 68.6 percent of participants showed improvement. N = number of participants.
Apimeds Korea used data from its first Phase III clinical trial for OA and peer reviewed publications, including those referenced in Table A above and formal Phase I (the “Castro Phase I Trial”) and Phase II (the “Wesselius Phase II Trial”) publications specific to MS, to support its submission in 2014 of its Investigational New Drug Application (“IND”) 122804 (A Phase III, Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Safety and Efficacy of Apitox Add-on Therapy for Improving Disability and Quality of Life in Patients with Multiple Sclerosis).
16
Castro Phase I Trial
The Castro Phase I Trial involved a total of nine bee venom nonallergic patients with progressive forms of MS, who were 21 – 55 years of age with no other illnesses. The subjects distributed across four groups (A, B, C, and D) and followed a structured 1-year immunization schedule. Hyperreactivity to bee venom was evaluated by questionnaire, physical examination, and a battery of hematologic, metabolic, and immunologic tests. Responses to therapy were evaluated by questionnaire, functional neurological tests, and changes in measurement of somatosensory-evoked potentials. While no serious adverse allergic reactions were observed in any of the subjects, four experienced worsening of neurological symptoms, requiring their discontinuation in the study. The observed negative effects could not be conclusively attributed to adverse reactions arising from the administered therapy. Of the remaining five subjects, three reported subjective amelioration of symptoms and two exhibited objective improvement. Despite suggesting safety in this preliminary study, the small sample size precluded definitive conclusions regarding the efficacy of the treatment for MS. Larger and more carefully conducted multicenter studies were required to establish efficacy.
Wesselius Phase II Trial
The Wesselius Phase II Trial involved a randomized crossover study of 26 patients diagnosed with relapsing-remitting or relapsing secondary progressive MS. Participants were assigned to 24 weeks of medically supervised bee sting therapy, or a control period of 24 weeks of no treatment. Live bees (up to a maximum of 20) were used to administer bee venom three times per week. The primary outcome was the cumulative number of new gadolinium-enhancing lesions on T1-weighted MRI of the brain. Secondary outcomes were lesion load on T2*-weighted MRI, relapse rate, disability (Expanded Disability Status Scale, Multiple Sclerosis Functional Composite, Guy’s Neurologic Disability Scale), fatigue (Abbreviated Fatigue Questionnaire, Fatigue Impact Scale), and health-related quality of life (Medical Outcomes Study 36-Item Short Form General Health Survey). The results of the Wesselous Phase II Trial indicated that during bee sting therapy, there was no significant reduction in the cumulative number of new gadolinium-enhancing lesions. The T2*-weighted lesion load further progressed, and there was no significant reduction in relapse rate. There was no improvement of disability, fatigue, and quality of life. Bee sting therapy was well tolerated, and there were no serious adverse events. In this trial, treatment with bee venom in patients with relapsing multiple sclerosis did not reduce disease activity, disability, or fatigue and did not improve quality of life measured using gadolinium-enhancing MRI.
From June 2014 to June 2018, Apimeds Korea corresponded with the FDA and there were no clinical holds at that time. Sponsorship of IND 122804 was transferred from Apimeds Korea to us in October 2020. On September 21, 2021, we responded to customary non-clinical hold comments from the FDA. In November 2021, we received a customary clinical hold from the FDA due to the retirement of the former principal investigator. We have subsequently updated the FDA with a new principal investigator via our Chief Medical Officer, Dr. Christopher Kim. In February 2023, the FDA removed the clinical hold and concluded it may be initiated. We have subsequently made the strategic decision to focus our efforts and capital on our Phase III trial in knee OA, and instead focus our MS efforts on the early prosecution of appropriate MS patient populations through non-registered corporate sponsorship studies.
Our Commercialization Strategy
We are dedicated to the effective implementation of regulatory, clinical and legal strategies to create value in Apitox. The effective execution of this strategy will provide us the opportunity to evaluate and potentially acquire other assets that fit within our space for development.
17
Manufacturing
We intend to continue to engage a third-party manufacturer, Piramal Pharma Solutions, in Lexington, Kentucky to support our Phase III trial and, if Apitox is approved by the FDA, commercial manufacturing. This manufacturer has dedicated experience in development and technology transfer of sterile dose formulations, including liquid and lyophilized formulations.
Research and Development
We are currently engaged exclusively in the clinical development of Apitox for continued use in knee OA through a Phase III trial in knee OA and potential use for MS through the early prosecution of appropriate patient populations through non-registered corporate sponsorship studies.
Sales and Marketing
The healthcare providers associated with the treatment of inflammation and pain management symptoms associated with OA and MS are not limited to one specialist but involve a comprehensive team of providers focused on slowing the progression of the disease along with the physical, emotional and day-to-day management of the condition. Each of these providers represents a potential customer for Apitox.
Apitoxin, which will be known as Apitox in the United States, has established technological credibility through its preclinical testing, Phase I, Phase II and preliminary Phase III clinical studies completed by Apimeds Korea. Apimeds Korea received regulatory approval for Apitoxin by the MFDA in South Korea, as well as long-term safety data from treatment of patients in Korea from 2003 to 2009. There were no serious adverse events from over 3,000 patients monitored, and Apitoxin has been approved and marketed in South Korea for OA since 2003. We update the FDA annually on safety data generated by Apimeds Korea from South Korea.
We aim to obtain FDA approval for Apitox in the United States market for treatment of inflammation and pain management symptoms associated with knee OA, and eventually MS, and expand the indication portfolio in the autoimmune market with a strategic marketing partner. The marketing partner strategy is common in the pharmaceutical marketplace, as the infrastructure, overhead, and barriers to entry dilute the focus and can rapidly erode the financial well-being of small, product development-based companies such as us. By identifying the strategic marketing partner at an early stage, the companies can deliver a final product, or family of products, in a form factor or variety of form factors over time, that specifically suit the target market. We believe that Apitox represents a significant opportunity as a platform technology, with numerous product-line extensions, and the potential for new, ancillary products such as delivery devices.
Reimbursement Strategy
Apimeds expects to apply to the Centers for Medicare and Medicaid Studies (“CMS”) for temporary generic reimbursement codes 12 to 18 months prior to a BLA approval. Temporary codes are used until manufacturers apply for, and receive, permanent codes, which identify the drug and its therapeutic class. Permanent codes are issued by CMS on a rolling quarterly basis.
We will engage third party contractors to assist the us with reimbursement, coding and policy development prior to, during and at the time of approval of Apitox. We will look for a contractor to provide the following services to us:
• Coding Assessment and Strategy/Execution — CPT Review of Apitox Administration by Multiple Intradermal Injections. Assess the landscape to ensure a clear understanding of the key dynamics and analyze relevant proxies and precedent. Further assess relevant drug administration codes and whether appropriate codes exist.
• Medical Coverage Policy Analysis — Provide a framework and set expectations for Medicare’s anticipated coverage approach to Apitox, specifically in the context of intra articular hyaluronic acid use agent coverage policies and implications of their efficacy uncertainty.
18
• Medicare Local Coverage Analysis and Implications — Given the significance of Medicare policy standards, local and national Medicare policies often shape payer and provider perceptions and decisions. As complex statutory and regulatory guidance shape Medicare decision-making, ADVI analyzes, investigates, and synthesize Medicare policies that could affect access (coverage, coding and reimbursement) for Apitox.
• Medicaid and Commercial Coverage Analysis and Implications — Analyze available medical policies for five large state Medicaid agencies (based on population and geographic variation) and major commercial payers (where publicly available).
• Payer Policy Internal Expert Interviews — Conduct payer interviews with relevant Medicare, Medicaid and commercial policy advisors.
• HCPCS Coding and Payment Assessment — Assess the coding and reimbursement landscape to ensure Apimeds has a clear understanding of the key dynamics with the HCPCS application process and the Medicare Hospital Outpatient Prospective Payment System (OPPS) pass-through status application process. Through this assessment, identify the areas of concern, expectations, timing, timelines, and processes associated. This is especially relevant given the 2020 implementation of a new HCPCS review process.
• Address key Part B/medical benefit implications to Apitox in the following fields:
• HCPCS and OPPS application timelines (and potential evolution leading to launch),
• coding/access implications prior to code assignment (e.g., NOC/miscellaneous codes), review the merits/risks of Q-code,
• further review the application processes, expectations, case examples, timelines, and hurdles that APUS may face across settings of care, payers, and with CMS,
• case examples, timelines, and hurdles across settings of care with payers and CMS,
• review of reimbursement implications, and
• methodologies (ASP, WAC, AWP), role of sequestration, 340B, patient financial burden.
• Develop Payer (with Emphasis on Medicare) Launch Recommendations — Based on the above primary and secondary research, synthesize the discussions and summarize the overall findings of the payer survey, highlighting themes, and provide recommendations and considerations for optimizing market access, given the current and evolving reimbursement landscape. This section will include payer (emphasis on Medicare) launch strategy recommendations (including timeline) and a local/national Medicare engagement strategy.
Competition
We compete in an industry characterized by rapidly advancing technologies, intense competition, a changing regulatory and legislative landscape and a strong emphasis on the benefits of intellectual property protection and regulatory exclusivities.
Like any biopharmaceutical company, we face competition from multiple sources, including large or established pharmaceutical, biotechnology, and wellness companies, academic research institutions, government agencies, and private institutions. We believe our drug candidate will prevail amid the competitive landscape through its efficacy, safety, administration methods, cost, public and institutional demand, intellectual property portfolio, and treatment of the root cause of many age-associated diseases.
Many of our competitors, either alone or with strategic partners, have substantially greater financial, technical, and human resources than we do. Accordingly, our competitors may be more successful in obtaining approval for treatments and achieving widespread market acceptance, rendering our treatments obsolete or non-competitive. Accelerated merger and acquisition activity in the biotechnology and biopharmaceutical industries may result in even more resources concentrated among a smaller number of our competitors. These companies also compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical study sites, patient
19
registration for clinical studies, and acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Our commercial opportunity could be substantially limited in the event that our competitors develop and commercialize products that are more effective, safer, more tolerable, more convenient, or less expensive than our comparable products. In geographies that are critical to our commercial success, competitors may also obtain regulatory approvals before us, resulting in our competitors building a strong market position in advance of our products’ entry. We believe the factors determining the success of our programs will be the efficacy, safety, and convenience of our drug candidates.
Additionally, consumer preference for branded, generic or private label products sold by competitors could adversely impact our financial performance. Our competitors, which differ within individual geographic markets, include large-scale retailers, smaller high-growth companies (which often operate on a regional basis and offer aggressive competition), multinational corporations moving into or expanding their presence in the consumer healthcare market, and “private-label” products sold by retailers.
Our aim is to reduce the use of NSAIDS and opioid use as it relates to the pain management associated with OA. We believe that if approved by the FDA, Apitox may be a non-addictive option to patients experiencing debilitating pain.
Business Agreement
On August 2, 2021, we entered into an agreement with Apimeds Korea, a principal stockholder of the Company (the “Business Agreement”). Pursuant to the Business Agreement, Apimeds Korea granted to the Company a sublicensable, royalty-bearing license to utilize all prior clinical development data associated with Apitoxin, Apitox, and all related names, advance clinical research, develop, manufacture and commercialize and sell Apitox in the United States. In exchange for this license, the Company will pay Apimeds Korea a perpetual royalty of 5% of the Company’s earnings before interest and taxes (as determined consistent with GAAP, derived from the sale or license of Apitox, less any shipping, handling, and insurance charges, credits (arising from returns or other adjustments), discounts, rebates, or allowances of any kind (if any). The Business Agreement can be terminated by mutual written agreement by the parties and will automatically terminate upon the bankruptcy or dissolution of the Company.
Assignment Agreement
On October 12, 2021, we entered into an intellectual property assignment agreement (the “Assignment Agreement”), which was effective as of May 12, 2020, with Apimeds Korea and Dr. Christopher Kim, the Company’s Chairman and Chief Medical Officer and the founder of Apimeds Korea. During Dr. Kim’s engagement with Apimeds Korea, he contributed to the development of the intellectual property as it relates to Apitoxin, which will be marketed in the United States as Apitox (the “Assigned IP”).
Pursuant to the Assignment Agreement, Dr. Kim sold, transferred, and conveyed all his rights, title and interest in the Assigned IP to Apimeds Korea. Dr. Kim retained no right to use the Assigned IP. Additionally, the Assignment Agreement acknowledged that the Assigned IP was licensed to us to use via the Business Agreement.
Intellectual Property
Apitox’s API is bee venom, a natural, non-synthetic compound that is not patentable, so we rely principally on trade secrets to protect our rights to Apitox, particularly the method and process of manufacturing Apitox.
Supplier
We purchase venom from our United States supplier, Apico, Inc. (“Apico”), via a letter agreement. Pursuant to the letter agreement, Apico agreed that for a period of ten years, or until November 3, 2031 it would not supply Apis Mellifea venom for pharmaceutical use for any buyer other than us; provided that Apico may also supply Apimeds Korea for its use outside of the United States. The letter agreement excludes customers using venom for immunology, cosmetic or any other “non-pharmaceutical” use. The letter agreement may be terminated upon mutual written consent of both Apico and the Company.
Apico has developed and practices a proprietary method of harvesting venom. It operates under and is certified in current good manufacturing practice regulations enforced by the FDA and has an active and current Drug Master
20
File (“DMF”) with the FDA. DMF’s are submissions to the FDA used to provide confidential, detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of human drug products. We have an exclusive relationship with our supplier for pharmaceutical use in the United States and they are not permitted to sell to any other party for pharmaceutical use.
Apimeds Korea has a number of proprietary analytical methods for the classification and identification of specific pharmacologically active fractions of its venom, along with numerous manufacturing processes from filtration, vial filing and lyophilization required to produce Apitoxin. Apitoxin is the only approved and commercially available therapeutic product containing purified and sterile bee venom that is registered as an API in South Korea. The proprietary methods developed and practiced for the commercial manufacturing of Apitoxin include dilution, filtering, vial staging and lyophilization parameters and cycles.
We plan to file Apitox as a BLA with the Centers for Biologics and Research of the FDA following the successful completion of our Phase III trial for knee OA. The FDA provides 12-year market exclusivity at the time of approval of a BLA, with the potential for a six-month extension upon approval for pediatric use. If the BLA is approved, the 12-year period would be retroactive to the date of the application.
We intend to file a U.S. trademark application for “Apitox”.
Regulatory Environment
Government Regulation and Product Approval
In the United States, biological products are subject to regulation under the Federal Food, Drug, and Cosmetic Act (the “FDCA”), and the Public Health Service Act (the “PHSA”), and other federal, state, and local statutes and regulations. Both the FDCA and PHSA and their corresponding regulations govern, among other things, the research, development, clinical trials, testing, manufacturing, quality control, safety, purity and potency (efficacy), labeling, packaging, storage, record keeping, distribution, reporting, marketing, promotion, advertising, post-approval monitoring, and post-approval reporting involving biological products. Along with third-party contractors, we will be required to navigate the various preclinical and clinical regulatory obligations and the commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of our product candidate. The processes for obtaining regulatory approvals in the United States, along with subsequent compliance with applicable laws and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources.
Government policies may change, and additional government regulations may be enacted that could prevent or delay further development or regulatory approval of any product candidates, product or manufacturing changes, additional disease indications or label changes. We cannot predict the likelihood, nature or extent of government regulation that might arise from future legislative or administrative action.
Review and Approval for Licensing Biologics in the United States
In the United States, FDA regulates our current product candidate as a biological product, or biologics, under the FDCA, the PHSA, and associated implementing regulations. Biologics, like other drugs, are used for the diagnosis, cure, mitigation, treatment, or prevention of disease in humans. In contrast to low molecular weight drugs, which have a well-defined structure and can be thoroughly characterized, biologics are generally derived from living material (human, animal, or microorganism), are complex in structure, and thus are usually not fully characterized.
Biologics are also subject to other federal, state, and local statutes and regulations. The failure to comply with applicable statutory and regulatory requirements at any time during the product development process, approval process, or after approval may subject a sponsor or applicant to administrative or judicial enforcement actions. These actions could include the suspension or termination of clinical trials by FDA, FDA’s refusal to approve pending applications or supplemental applications, withdrawal of an approval, issuance of warning or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, import detention, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations and penalties brought by FDA, the Department of Justice (“DOJ”), and other governmental entities.
21
An applicant seeking approval to market and distribute a biologic in the United States must typically undertake the following:
• completion of non-clinical laboratory tests and studies performed in accordance with FDA’s good laboratory practice (“GLP”) regulations;
• manufacture, labeling and distribution of investigational drugs in compliance with FDA’s current good manufacturing practice (“cGMP”) requirements;
• submission to FDA of an investigational new drug application (“IND”), which must become effective before clinical trials may begin and must be updated annually and when significant changes are made;
• approval by an independent institutional review board (“IRB”) for each clinical site before each clinical trial may be initiated;
• performance of adequate and well-controlled human clinical trials in accordance with FDA’s Good Clinical Practices (“GCP”) to establish the safety, purity, and potency of the proposed biological product candidate for its intended purpose;
• after completion of all pivotal clinical trials, preparation of and submission to FDA of a BLA requesting marketing approval, which includes providing sufficient evidence to establish the efficacy, safety, purity, and potency of the proposed biological product for its intended use, including from results of nonclinical testing and clinical trials;
• satisfactory completion of an FDA advisory committee review, when appropriate, as may be requested by FDA to assist with its review;
• satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the proposed product, or certain components thereof, are produced to assess compliance with cGMP and data integrity requirements to assure that the facilities, methods, and controls are adequate to preserve the biological product’s identity, strength, quality, and purity and, if applicable, FDA’s good tissue practice (“GTP”) requirements for human cellular and tissue products;
• satisfactory completion of FDA inspections of selected clinical investigation sites to assure compliance with GCP requirements and the integrity of the clinical data;
• satisfactory completion of an FDA sponsor GCP inspection, often conducted at the applicant’s headquarters facility;
• payment of user fees (unless there is a waiver, exemption, or reduction) under the Prescription Drug User Fee Act (“PDUFA”) for the relevant year;
• FDA’s review and approval of the BLA to permit commercial marketing of the licensed biologic for particular indications for use in the United States;
• compliance with post-approval requirements, including the potential requirements to implement a risk evaluation and mitigation strategy (“REMS”), to report adverse events and biological product deviations, and to complete any post-approval studies; and
• completion of any post-approval clinical studies required by FDA, such as confirmatory trials or pediatric studies.
From time to time, legislation is drafted, introduced, and passed in Congress that could significantly change the statutory provisions governing the testing, approval, manufacturing, and marketing of biological products regulated by FDA. In addition to new legislation, FDA regulations, guidance documents, and policies are often revised or interpreted by the agency in ways that may significantly affect the regulation of biological products in the United States. It is impossible to predict whether further legislative changes will be enacted or whether FDA regulations, guidance, policies, or interpretations will change, and the effects of any such changes.
22
Preclinical and Clinical Development
Before an applicant can begin testing the potential product candidate in human subjects, the applicant must first conduct preclinical studies. Preclinical studies may include laboratory evaluations of product chemistry, toxicity, and formulation, as well as in vitro and animal studies to assess the potential safety and activity of the drug for initial testing in humans and to establish a rationale for therapeutic use. Preclinical studies are subject to federal regulations and requirements, including GLP regulations, which govern the conduct of animal studies designed to test a product’s safety. None of our preclinical studies to date have been animal studies. The results of an applicant’s preclinical studies are submitted to FDA as part of an IND.
An IND is a request for authorization from FDA to administer an investigational new drug product to humans. An IND is an exemption from the FDCA that allows an unapproved drug to be shipped in interstate commerce for use in a clinical trial. Such authorization must be secured prior to interstate shipment and administration of a biological drug that is not subject of an approved BLA. In support of an IND, applicants must submit a protocol for each clinical trial, which details, among other things, the objectives of the trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments.
Human clinical trials may not begin until an IND is effective. The IND automatically becomes effective 30 days after receipt by FDA, unless FDA raises safety concerns or questions about the proposed clinical trial within the 30-day time period. In such a case, FDA may place the IND on clinical hold and the IND sponsor must resolve any of FDA’s outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in regulatory authorization to begin a clinical trial.
FDA may also place a clinical hold or partial clinical hold on a clinical trial following commencement of the trial under an IND. A clinical hold is an order issued by FDA to the sponsor to delay a proposed clinical investigation or to suspend an ongoing investigation. A partial clinical hold is a delay or suspension of only part of the clinical work requested under the IND. For example, under a partial clinical hold, FDA may instruct a sponsor not to enroll any new patients into a study but permit the previously enrolled patients to continue in the study. No more than 30 days after imposition of a clinical hold or partial clinical hold, FDA will provide the sponsor a written explanation of the basis for the hold. Following issuance of a clinical hold or partial clinical hold, an investigation may only resume after the FDA has notified the sponsor that the investigation may proceed. FDA will base that determination on information provided by the sponsor addressing the deficiencies previously cited or otherwise satisfying FDA that the investigation can proceed.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCP regulations, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. If a sponsor chooses to conduct a foreign clinical study under an IND, all FDA IND requirements must be met unless waived. When the foreign clinical study is not conducted under an IND, the sponsor must ensure that the study complies with GCP regulations in order to use the study as support for an IND or application for marketing approval, including review and approval by an IRB and informed consent from subjects.
Furthermore, an independent IRB for all sites participating in a clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at each site and must monitor the trial until completed. Regulatory authorities, the IRB, or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives.
Some trials also include oversight by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board (“DSMB”). DSMBs review unblinded study data at pre-specified times during the course of the study. If the DSMB determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy, the DSMB can make a recommendation to the sponsor to modify or stop the trial.
Other grounds for a sponsor’s decision to suspend or terminate a study may be made based on evolving business objectives or the competitive climate.
23
For purposes of BLA approval, clinical trials are typically conducted in the following sequential phases:
• Phase 1: The investigational product is initially introduced into a small group of healthy human subjects or patients with the target disease or condition. These trials are designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the investigational product in humans and the side effects associated with increasing doses. These trials may also yield early evidence of effectiveness.
• Phase 2: The investigational product is administered to a slightly larger patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages, and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase III clinical trials.
• Phase 3: The investigational product is administered to an expanded patient population to further evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to generate sufficient data to statistically demonstrate the efficacy and safety of the product, to establish the overall risk/benefit ratio of the investigational product, and to provide an adequate basis for product approval by FDA.
These phases may overlap or be combined. In some cases, FDA may require, or companies may voluntarily pursue, additional clinical trials after a product are approved to gain more information about the product, referred to as Phase 4 trials. Post-approval trials are conducted following initial approval, often to develop additional data and information relating to the use of the product in new indications.
Progress reports detailing the results of the clinical trials must be submitted at least annually to FDA. In addition, IND safety reports must be submitted to FDA for any of the following: serious and unexpected suspected adverse reactions in study subjects; findings from epidemiological studies, pooled analysis of multiple studies, animal or in vitro testing, or other clinical studies, whether or not conducted under an IND, and whether or not conducted by the sponsor, that suggest a significant risk in humans exposed to the drug; and any clinically important increase in the rate of a serious suspected adverse reaction over such rate listed in the protocol or investigator brochure.
A sponsor’s planned clinical trials may not be completed successfully within any specified period, or at all. Furthermore, the FDA or the sponsor may suspend or terminate a clinical trial at any time on various grounds, including a finding that the research subjects are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution, or an institution it represents, if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. FDA will typically inspect one or more clinical sites to assure compliance with GCP and the integrity of the clinical data submitted.
During clinical development, the sponsor often refines the indication and endpoints on which the BLA will be based. For endpoints based on patient-reported outcomes (“PROs”), the process typically is an iterative one. FDA has issued guidance on the framework it uses to evaluate PRO instruments. Although the agency may offer advice on optimizing PRO instruments during the clinical development process, FDA usually reserves final judgment until it reviews the BLA.
Concurrent with clinical trials, companies often complete additional animal studies, and develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and, among other things, must develop methods for testing the identity, strength, quality, purity and potency of the final drug. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf life.
BLA Submission and Review
Assuming successful completion of all required clinical testing in accordance with all applicable regulatory requirements, an applicant may submit a BLA requesting licensing to market the biologic for one or more indications in the United States. The BLA must include the results of nonclinical studies and clinical trials; detailed information on the product’s chemistry, manufacture, controls; and proposed labeling. Under the PDUFA, a BLA submission is subject to an application user fee, unless a waiver, reduction, or exemption applies.
24
FDA will initially review the BLA for completeness before accepting it for filing. Under FDA’s procedures, the agency has 60 days from its receipt of a BLA to determine whether the application will be accepted for filing and substantive review. If the agency determines that the application does not meet this initial threshold standard, FDA may refuse to file the application and request additional information, in which case the application must be resubmitted with the requested information and review of the application delayed.
After the BLA is accepted for filing, FDA reviews the BLA to determine, among other things, whether a product is safe, pure, and potent and if the facility in which it is manufactured, processed, packed, or held meets standards designed to assure the product’s continued identity, strength, quality, safety, purity, and potency. To ensure cGMP, GLP, GCP, GTP, and other regulatory compliance, an applicant must incur significant expenditure of time, money, and effort in the areas of training, record keeping, production and quality control. In addition, FDA expects that all data be reliable and accurate, and requires sponsors to implement meaningful and effective strategies to manage data integrity risks. Data integrity is an important component of the sponsor’s responsibility to ensure the safety, efficacy and quality of its product or products.
For cellular products, FDA will not approve the product if the manufacturer is not in compliance with the GTPs, to the extent applicable. GTPs are FDA regulations and guidance documents that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissue, and cellular and tissue-based products (“HCT/Ps”), which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the GTP requirements is to ensure that cell and tissue-based products are manufactured in a manner designed to prevent the introduction, transmission and spread of communicable disease. FDA regulations also specify how HCT/P establishments must register and list their HCT/Ps with FDA and how they must evaluate donors through screening and testing, where applicable.
If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The performance goals and policies implemented by FDA under the PDUFA generally provide for FDA action on an original BLA within 10 months of filing, which (as discussed above) typically occurs within 60 days of submission, but that deadline is extended in certain circumstances. Furthermore, the review process is often significantly extended by FDA’s requests for additional information or clarification.
FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee. Typically, an advisory committee consists of a panel that includes clinicians and other experts who will review, evaluate, and provide a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions and usually has followed such recommendations.
After FDA evaluates a BLA and conducts inspections of manufacturing facilities where the investigational product and/or its components will be produced, FDA may issue an approval letter or a Complete Response Letter (“CRL”). An approval letter authorizes commercial marketing of the biological with specific prescribing information for specific indications. A CRL will describe all of the deficiencies that FDA has identified in the BLA, except that where FDA determines that the data supporting the application are inadequate to support approval, FDA may issue the CRL without first conducting required inspections, testing submitted product lots and/or reviewing proposed labeling. If and when the deficiencies have been addressed to FDA’s satisfaction in a resubmission of the BLA, FDA will issue an approval letter. In issuing the CRL, the FDA may recommend actions that the applicant might take to place the BLA in condition for approval, including requests for additional data, information, or clarification. FDA may delay or refuse approval of a BLA if applicable regulatory criteria are not satisfied and may require additional testing or information and/or require new clinical trials. Even with submission of this additional information, FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
During the approval process, FDA will determine whether a REMS is necessary to help ensure the benefits outweigh the risks of the biologic. A REMS is a safety strategy to manage a known or potential serious risk associated with a product and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans or elements to assure safe use, such as restricted distribution
25
methods, patient registries and other risk minimization tools. If FDA concludes that a REMS is needed, the BLA sponsor must submit a proposed REMS and FDA will not approve the BLA without a REMS that the agency has determined is acceptable.
If the FDA approves a product, it may limit the approved indications for use for the product, or require that contraindications, warnings, or precautions be included in the product labeling. FDA may also require that post-approval studies, including Phase 4 clinical trials, be conducted to further assess the drug’s safety after approval. FDA may prevent or limit further marketing of a product based on the results of post-market studies or surveillance programs.
FDA may also require testing and surveillance programs to monitor the product after commercialization. For biologics, such testing may include official lot release, which requires the manufacturer to perform certain tests on each lot of the product before it is released for distribution. The manufacturer then typically must submit samples of each lot of products to the FDA, together with a release protocol showing a summary of the history of manufacture of the lot and the results of all of the manufacturer’s tests performed on the lot. The FDA may also perform certain confirmatory tests on lots of some products itself, before releasing the lots for distribution by the manufacturer.
In general, an approved BLA only allows the sponsor to market the biologic as approved, without modification. If, for example, a sponsor modifies an approved T cell product to target different peptides or in our case to target another HLA type, the sponsor would be required to either file a supplemental BLA with FDA or receive FDA approval for a comparability protocol in order to implement this change into the final product.
The FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace.
Post-Approval Requirements
Any products manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by FDA, including, among other things, requirements relating to recordkeeping, periodic reporting, reporting of certain deviations and adverse experiences, product sampling and distribution, and advertising and promotion of the product. After approval, many types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are often subject to further testing requirements and FDA review and approval, depending on the nature of the post-approval change. There also are continuing user fee requirements, under which FDA assesses an annual program fee for each product identified in an approved BLA. Biologic manufacturers and their third-party contractors are required to register their facilities with the FDA and certain state agencies. These facilities are subject to routine and periodic unannounced inspections by FDA and certain state agencies for compliance with cGMP, post-marketing safety reporting and data integrity requirements, which impose certain procedural and documentation requirements to assure quality of manufacturing and product. FDA has increasingly observed cGMP violations involving data integrity during site inspections and is a significant focus of its oversight. Requirements with respect to data integrity include, among other things, controls ensuring complete and secure data; activities documented at the time of performance; audit trail functionality; authorized access and limitations; validated computer systems; and review of records for accuracy, completeness, and compliance with established standards.
Post-approval changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting requirements upon the sponsor and any third-party manufacturers that the sponsor may use. Accordingly, manufacturers must continue to expend time, money, and effort in the area of production and quality control to maintain compliance with cGMP, data integrity, pharmacovigilance, and other aspects of regulatory compliance.
The FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-approval studies to assess new safety risks; or imposition of distribution or other restrictions under a REMS. Other potential consequences include, for example:
• restrictions on the marketing or manufacturing of a product, complete withdrawal of the product from the market, or product recalls;
26
• fines, warning or untitled letters, or holds on post-approval clinical studies;
• refusal of FDA to approve pending applications or supplements to approved applications, or suspension or revocation of existing product approvals;
• product seizure or detention, or refusal of FDA to permit the import or export of products; or
• permanent injunctions and consent decrees, including the imposition of civil or criminal penalties.
FDA strictly regulates the marketing, labeling, advertising, and promotion of prescription drug products placed on the market. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA and in accordance with the provisions of the approved labeling. FDA’s regulation includes, among other things, standards and regulations for direct-to-consumer advertising, communications regarding unapproved uses, industry-sponsored scientific and educational activities and promotional activities involving the Internet and social media. Promotional claims relating to a product’s safety or effectiveness are prohibited before the drug is approved. After approval, a product generally may not be promoted for uses that are not approved by FDA, as reflected in the product’s prescribing information. In the United States, healthcare professionals are generally permitted to prescribe drugs for such uses not described in the drug’s labeling, known as off-label uses, because FDA does not regulate the practice of medicine. However, FDA regulations impose rigorous restrictions on manufacturers’ communications and prohibit the promotion of off-label uses. It may be permissible, under very specific, narrow conditions, for a manufacturer to engage in non-promotional, non-misleading communication regarding off-label information, such as distributing scientific or medical journal information.
If a company is found to have promoted off-label uses, it may become subject to adverse public relations and administrative and judicial enforcement by FDA, the DOJ, or the Office of the Inspector General of the Department of Health and Human Services (“HHS”), as well as other federal and state authorities. This could subject a company to a range of penalties that could have a significant commercial impact, including civil, administrative, and criminal fines, penalties, and agreements that materially restrict the manner in which a company promotes or distributes products. The federal government has levied large civil, administrative, and criminal fines and penalties against companies for alleged improper promotion and has also requested that companies enter into Corporate Integrity Agreements and Consent Decrees of Permanent Injunction under which specified promotional conduct is changed or curtailed.
The distribution of prescription drugs and biologics are subject to the Drug Supply Chain Security Act (“DSCSA”), which requires manufacturers and other stakeholders to comply with product identification, tracing, verification, detection and response, notification, and licensing requirements. In addition, the Prescription Drug Marketing Act and its implementing regulations and state laws limit the distribution of prescription pharmaceutical product samples, and the DSCSA imposes requirements to ensure accountability in distribution and to identify and remove prescription drug and biological products that may be counterfeit, stolen, contaminated, or otherwise harmful from the market.
Expedited Development and Review Programs
FDA offers a number of expedited development and review programs for qualifying product candidates. The fast-track program is intended to expedite or facilitate the process of reviewing new products that meet certain criteria. Specifically, new products are eligible for fast-track designation if they are intended to treat a serious or life-threatening disease or condition and demonstrate the potential to address unmet medical needs for the disease or condition. A product intended to treat a serious or life-threatening disease or condition may also be eligible for breakthrough therapy designation to expedite its development and review. Any marketing application for a biologic submitted to FDA for approval, including a product with a fast-track designation and/or breakthrough therapy designation, may be eligible for other types of FDA programs intended to expedite FDA review and approval process, such as priority review and accelerated approval. FDA also may grant accelerated approval to certain products studied for their safety and effectiveness in treating serious or life-threatening diseases or conditions.
The RMAT designation, which we are currently planning to seek for some of our therapies, is intended to facilitate an efficient development program for, and expedite review of, any drug that meets the following criteria: (1) the drug is a cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product using such therapies or products, with limited exceptions; (2) the drug is intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition; and (3) preliminary clinical evidence indicates that the drug has the potential to address unmet medical needs for such a disease or condition. Like breakthrough therapy designation, RMAT
27
designation provides potential benefits that include more frequent meetings with FDA to discuss the development plan for the product candidate and eligibility for rolling review and priority review. Products granted RMAT designation may also be eligible for accelerated approval on the basis of a surrogate or intermediate endpoint reasonably likely to predict long-term clinical benefit, or reliance upon data obtained from a meaningful number of sites (including through expansion to additional sites) so as to remove any likelihood of site-specific or investigator-specific bias on the evidence of effectiveness. Once approved, when appropriate, FDA can permit fulfillment of post-approval requirements for RMATs receiving accelerated approval through the submission of clinical evidence, clinical studies, patient registries, or other sources of real-world evidence such as electronic health records; through the collection of larger confirmatory datasets; or through post-approval monitoring of all patients treated with the therapy prior to approval.
Fast track designation, breakthrough therapy designation, priority review, accelerated approval, and RMAT designation do not change the standards for approval but may expedite the development or approval process.
Patent Term Restoration and Marketing Exclusivity
After approval, owners of relevant drug or biological product patents may apply for up to a five year term patent extension to restore a portion of patent term lost during product development and FDA review of a BLA if approval of the application is the first permitted commercial marketing or use of a drug or biologic containing the active ingredient under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Act. The allowable patent term extension is calculated as one-half of the product’s testing phase, which is the time between the effective date of an IND and initial BLA submission, and all of the approval phase, which is the time between BLA submission and approval, up to a maximum of five years. The time can be shortened if the FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years from the date of FDA approval of the product. Only one patent claiming each approved product is eligible for restoration and the patent holder must apply for restoration within 60 days of approval, even if the product cannot be commercially marketed at that time. The USPTO, in consultation with FDA, reviews and approves the application for patent term restoration.
For patents that might expire during the BLA application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the USPTO must determine that approval of the product candidate covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a product candidate for which a BLA has not been submitted.
Biosimilars and Marketing Exclusivities
The Biologics Price Competition and Innovation Act (“BPCIA”) created an abbreviated approval pathway for biological product candidates shown to be highly similar to or interchangeable with an FDA licensed biological product. A biological product on which another biological product candidate’s BLA relies to establish bio similarity is known as a reference product. Bio similarity sufficient to reference a prior FDA-approved product requires that there be no differences in conditions of use, route of administration, dosage form and strength, and no clinically meaningful differences between the biological product candidate and the reference product in terms of safety, purity, and potency. Bio similarity must be shown through analytical trials, animal trials and at least one clinical trial, unless the Secretary of HHS waives a required element. A biosimilar product candidate may be deemed interchangeable with a prior approved product if it meets the higher hurdle of demonstrating that it can be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biological product candidate and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. Complexities associated with the larger, and often more complex, structures of biologics, as well as the process by which such products are manufactured, pose significant hurdles to implementation of the abbreviated approval pathway that are still being resolved by FDA.
A reference biologic is granted 12 years of exclusivity from the time of first licensure of the reference product, and no application for a biosimilar can be submitted for four years from the date of licensure of the reference product. The first biological product candidate submitted under the abbreviated approval pathway that is determined to be interchangeable with the reference product has exclusivity against a finding of interchangeability for other biologics for the same
28
condition of use for the lesser of (i) one year after first commercial marketing of the first interchangeable biosimilar, (ii) 18 months after the first interchangeable biosimilar is approved if there is no patent challenge, (iii) 18 months after resolution of a lawsuit over the patents of the reference biologic in favor of the first interchangeable biosimilar applicant, or (iv) 42 months after the first interchangeable biosimilar’s application has been approved if a patent lawsuit is ongoing within the 42 month period. At this time, it is unclear whether products deemed “interchangeable” by FDA will, in fact, be readily substituted by pharmacies, which are governed by state pharmacy laws and regulations.
Healthcare Regulation
Coverage, Pricing, and Reimbursement
Our ability to successfully commercialize any products for which we receive regulatory approval for commercial sale will depend, in part, on the extent to which third-party payors provide coverage and establish adequate reimbursement levels for such products, and significant uncertainty exists as to the coverage and reimbursement status of any products for which may we obtain regulatory approval. In the United States, third-party payors include federal and state health care programs, private managed care providers, health insurers and other organizations. The process for determining whether a third-party payor will provide coverage for a product may be separate from the process for setting the price of a product or for establishing the reimbursement rate that such a payor will pay for the product. Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the FDA-approved products for a particular indication. Third-party payors are increasingly challenging the price, examining the medical necessity, and reviewing the cost-effectiveness of medical products, therapies, and services, in addition to questioning their safety and efficacy. We may need to conduct expensive pharmaco-economic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain the FDA approvals. Our product candidates may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage for the product. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
The marketability of any product candidates for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on healthcare pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
Although we currently do not have any commercialized products, our current and future business operations may be subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which we conduct our business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security, price reporting and physician sunshine laws. Some of our pre-commercial activities are subject to some of these laws.
The federal Anti-Kickback Statute makes it illegal for any person or entity, including a prescription drug manufacturer or a party acting on its behalf to knowingly and willfully, directly or indirectly, solicit, receive, offer, or pay any remuneration in cash or in kind that is intended to induce or reward the referral of business, including the purchase, order, or lease of any item or service for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, formulary managers and beneficiaries on the other.
Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead,
29
the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have found that the Anti-Kickback Statute may be violated if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare program business. In addition, liability may be established without actual knowledge of the statute or specific intent to violate it. Violations of this law are punishable by up to ten years in prison, and can also result in criminal fines, civil money penalties and exclusion from participation in federal healthcare programs.
Moreover, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal civil False Claims Act prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using, or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. Persons and entities can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. Many pharmaceutical and other healthcare companies have been investigated and have reached substantial financial settlements with the federal government under the civil False Claims Act for a variety of alleged improper marketing activities, including: providing free product to customers with the expectation that the customers would bill federal programs for the product; providing sham consulting fees, grants, free travel and other benefits to physicians to induce them to prescribe the company’s products; and inflating prices reported to private price publication services, which are used to set drug payment rates under government healthcare programs. Penalties for federal civil False Claims Act violations may include up to three times the actual damages sustained by the government, plus mandatory civil penalties of between $13,508 and $27,018 for each separate false claim, and the potential for exclusion from participation in federal healthcare programs. In addition, although the federal False Claims Act is a civil statute, False Claims Act violations may also implicate various federal criminal statutes.
The healthcare fraud provisions of the Health Insurance Portability and Accountability Act (“HIPAA”) prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Many states have analogous laws and regulations, such as: state anti-kickback and false claims laws that may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to certain healthcare providers; laws that require drug manufacturers to report information related to clinical trials or information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; laws that restrict the ability of manufacturers to offer co-pay support to patients for certain prescription drugs; and laws and local ordinances that require identification or licensing of sales representatives.
HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), and their implementing regulations, mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. Among other things, HITECH makes HIPAA’s security standards directly applicable to business associates, defined as independent contractors or agents of covered entities that create, receive, or obtain protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities and business associates and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil
30
actions. In addition, certain state laws govern the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties.
The U.S. federal Physician Payment Sunshine Act, implemented as the Open Payments Program, requires manufacturers of drugs, devices, biologics, and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to CMS information related to direct or indirect payments and other transfers of value to physicians and teaching hospitals (and certain other practitioners as of 2022), as well as ownership and investment interests held in the company by physicians and their immediate family members.
Because we intend to commercialize products that could be reimbursed under a federal health care program and other governmental healthcare programs, we intend to develop a comprehensive compliance program that establishes internal control to facilitate adherence to the rules and program requirements to which we will or may become subject. Although the development and implementation of compliance programs designed to establish internal control and facilitate compliance can mitigate the risk of investigation, prosecution, and penalties assessed for violations of these laws, the risks cannot be entirely eliminated.
If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and individual imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
Health Care Reforms
In the United States and some foreign jurisdictions, there have been, and continue to be, legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of product candidates, restrict or regulate post-approval activities, and affect the ability to profitably sell product candidates for which marketing approval is obtained. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives.
For example, the Affordable Care Act (“ACA”) substantially changed the way healthcare is financed by both the government and private insurers, and significantly impacts the U.S. pharmaceutical industry. The ACA contains provisions that may reduce the profitability of drug products through increased rebates for drugs reimbursed by Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries, and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. The ACA made several changes to the Medicaid Drug Rebate Program, including increasing pharmaceutical manufacturers’ rebate liability by raising the minimum basic Medicaid rebate. The ACA also expanded the universe of Medicaid utilization subject to drug rebates by requiring pharmaceutical manufacturers to pay rebates on Medicaid managed care utilization and by enlarging the population potentially eligible for Medicaid drug benefits.
There have been judicial challenges to certain aspects of the ACA, as well as efforts by Congress to modify, and by agencies to alter the implementation of, certain aspects of the ACA. For example, Congress eliminated the tax penalty for failure to comply with the ACA’s individual mandate to carry health insurance. Further, the Bipartisan Budget Act of 2018, among other things, amended the ACA to increase from 50 percent to 70 percent the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D to close the coverage gap in most Medicare drug plans, commonly referred to as the donut hole.
It is possible that the ACA, as currently enacted or as may be amended in the future, as well as other healthcare reform measures, including those that may be adopted in the future, may result in more rigorous coverage criteria, and less favorable payment methodologies, or other downward pressure on coverage and payment and the price that we receive for any approved product. Any reduction in reimbursement or restriction on coverage under Medicare or other federal health care programs may result in a similar reduction or restriction by private payors.
31
Other legislative changes have been proposed and adopted in the U.S. since the ACA was enacted. For example, the Inflation Reduction Act introduces several changes to the Medicare Part D benefit, including a limit on annual out-of-pocket costs and a change in manufacturer liability under the program which could negatively affect the profitability of our product candidates. The IRA sunsets the current Part D coverage gap discount program starting in 2025 and replaces it with a new manufacturer discount program. Failure to pay a discount under this new program will be subject to a civil monetary penalty. In addition, the IRA establishes a Medicare Part B inflation rebate scheme effective January 2023 and a Medicare Part D inflation rebate scheme effective October 2022, under which, generally speaking, manufacturers will owe rebates if the price of a Part B or Part D drug increases faster than the pace of inflation. Failure to timely pay a Part B or D inflation rebate is subject to a civil monetary penalty. The IRA also creates a drug price negotiation program under which the prices for Medicare units of certain high Medicare spend drugs and biologicals without generic or biosimilar competition will be capped by reference to, among other things, a specified non-federal average manufacturer price starting in 2026. Failure to comply with requirements under the drug price negotiation program is subject to an excise tax and/or a civil monetary penalty. Congress continues to examine various policy proposals that may result in pressure on the prices of prescription drugs with respect to the government health benefit programs and otherwise. The IRA or other legislative changes could impact the market conditions for our product candidates.
In general, there has been heightened governmental scrutiny over the manner in which drug manufacturers set prices for their commercial products, which has resulted in several Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug product pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drug products. At the state level, legislatures have increasingly passed legislation and implemented regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Drug Pedigree Laws
State and federal governments have proposed or enacted various drug pedigree laws which can require the tracking of all transactions involving prescription drugs from the manufacturer to the pharmacy (or other dispensing) level. Companies are required to maintain records documenting the chain of custody of prescription drug products beginning with the purchase of such products from the manufacturer. Compliance with these pedigree laws requires implementation of extensive tracking systems as well as heightened documentation and coordination with customers and manufacturers. While we fully intend to comply with these laws, there is uncertainty about future changes in legislation and government enforcement of these laws. Failure to comply could result in fines or penalties, as well as loss of business that could have a material adverse effect on our financial results.
Federal Regulation of Patent Litigation Settlements and Authorized Generic Arrangements
As part of the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, companies are required to file with the U.S. Federal Trade Commission (“FTC”) and the U.S. Department of Justice certain types of agreements entered into between brand and generic pharmaceutical companies related to the settlement of patent litigation or manufacture, marketing and sale of generic versions of branded drugs. This requirement could affect the manner in which generic drug manufacturers resolve intellectual property litigation and other disputes with brand pharmaceutical companies and could result generally in an increase in private-party litigation against pharmaceutical companies or additional investigations or proceedings by the FTC or other governmental authorities.
Other
The U.S. federal government, various states and localities have laws regulating the manufacture and distribution of pharmaceuticals, as well as regulations dealing with the substitution of generic drugs for branded drugs. Our operations are also subject to regulation, licensing requirements and inspection by the states and localities in which our operations are located or in which we conduct business.
32
Certain of our activities are also subject to FTC enforcement actions. The FTC enforces a variety of antitrust and consumer protection laws designed to ensure that the nation’s markets function competitively, are vigorous, efficient and free of undue restrictions. Federal, state, local and foreign laws of general applicability, such as laws regulating working conditions, also govern us.
In addition, we are subject to numerous and increasingly stringent federal, state and local environmental laws and regulations concerning, among other things, the generation, handling, storage, transportation, treatment and disposal of toxic and hazardous substances, the discharge of pollutants into the air and water and the cleanup of contamination. We are required to maintain and comply with environmental permits and controls for some of our operations, and these permits are subject to modification, renewal and revocation by the issuing authorities. Our environmental capital expenditures and costs for environmental compliance may increase in the future as a result of changes in environmental laws and regulations or increased manufacturing activities at any of our facilities. We could incur significant costs or liabilities as a result of any failure to comply with environmental laws, including fines, penalties, third-party claims and the costs of undertaking a clean-up at a current or former site or at a site to which our wastes were transported. In addition, we have grown in part by acquisition, and our diligence may not have identified environmental impacts from historical operations at sites we have acquired in the past or may acquire in the future.
Employees
As of the date of this Information Statement, we have [two] full time employees. We have no part-time employees and we engage one consultant. We believe that we maintain good relations with our employees.
33
APIMEDS PHARMACEUTICALS US, INC.’S MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
References in this section to “we,” “us” or the “Company” refer to Apimeds Pharmaceuticals US, Inc. References to our “management” or our “management team” refer to our officers and directors. The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the unaudited condensed financial statements and the notes thereto contained elsewhere in this Information Statement. Certain information contained in the discussion and analysis set forth below includes forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly from the results, expectations and plans discussed in these forward-looking statements.
Special Note Regarding Forward-Looking Statements
This Information Statement includes “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the Exchange Act that are not historical facts, and involve risks and uncertainties that could cause actual results to differ materially from those expected and projected. All statements, other than statements of historical fact included in this Information Statement including, without limitation, statements in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” regarding our financial position, business strategy and the plans and objectives of management for future operations, are forward-looking statements. Words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “would” and variations thereof and similar words and expressions are intended to identify such forward-looking statements. Such forward-looking statements relate to future events or future performance, but reflect management’s current beliefs, based on information currently available. A number of factors could cause actual events, performance or results to differ materially from the events, performance and results discussed in the forward-looking statements. For information identifying important factors that could cause actual results to differ materially from those anticipated in the forward-looking statements, please refer to the Risk Factors section of our Annual Report on Form 10-K for the year ended December 31, 2024, filed with the SEC on April 15, 2025 (the “Annual Report”) and the “Risk Factors” section of this Information Statement. Our securities filings can be accessed on the EDGAR section of the SEC’s website at www.sec.gov. Except as expressly required by applicable securities law, we disclaim any intention or obligation to update or revise any forward-looking statements whether as a result of new information, future events or otherwise.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the unaudited condensed financial statements and the notes thereto contained elsewhere in this Information Statement. Certain information contained in the discussion and analysis set forth below includes forward-looking statements that involve risks and uncertainties.
Overview
Apimeds Pharmaceuticals US, Inc. is a clinical stage biopharmaceutical company that is in the process of developing Apitox, a proprietary intradermally administered bee venom-based toxin. Our primary focus is to advance Apitox in the treatment of inflammatory conditions in the United States, specifically osteoarthritis (“OA”) and, eventually, multiple sclerosis (“MS”).
Apitox, is currently marketed and sold by Apimeds, Inc. in South Korea (“Apimeds Korea”) as “Apitoxin” for the treatment of inflammation and pain management symptoms associated with OA. There is an extensive history of use of bee venom, both in the United States and around the world, to assist with pain management. We believe that, in addition to knee OA and MS, Apitox has the potential to help manage difficult to control pain and inflammation issues, which we will explore in the future.
Our Product Candidate
Our product candidate Apitox is a purified, pharmaceutical grade venom of the Apis mellifera, or honeybee, which is classified by the U.S Food and Drug Administration (“FDA”) as an active pharmaceutical ingredient. Apimeds Korea has developed a proprietary method and process of turning extracted bee venom into a lyophilized powder for reconstitution prior to intradermal dose injections, which they sell in South Korea as Apitoxin. Apimeds Korea has exclusively licensed to us all rights to develop, commercialize, market and sell Apitoxin as “Apitox” in the United States in exchange for a sales royalty.
34
The success of the Company is dependent on obtaining the necessary regulatory approvals of its product candidates, marketing its products and achieving profitable operations. The continuation of the research and development activities and the commercialization of its products, if approved, are dependent on the Company’s ability to successfully complete these activities and to obtain additional financing through a combination of financing activities and operations. It is not possible to predict either the outcome of future research and development or commercialization programs, or the Company’s ability to fund these programs.
Financial Results
Since inception, Apimeds has incurred significant operating losses. For the three and nine months ended September 30, 2025 and 2024, Apimeds Pharmaceuticals US, Inc. net loss was $1,781,255 and $4,845,845 and $332,521 and $1,078,357, respectively.
Liquidity
As of September 30, 2025, the Company had accumulated deficit amount to $9,237,769 The Company incurred net losses of $1,781,255 and $4,845,845 for the three and nine months ended September 30, 2025, respectively, and expects to continue to incur substantial losses in the future. On May 12, 2025, the Company consummated its initial public offering (the “IPO”) of 3,375,000 shares of its common stock at a price of $4.00 per share, generating net proceeds to the Company of $11.9 million. Based on cash that is available for Company operations, together with the proceeds from the IPO, and projections of future Company operations, the Company believes that its cash will be sufficient to fund the Company’s current operating plan through at least the next twelve months from the date of issuance of the accompanying condensed financial statements.
Results of operations for the three months ended September 30, 2025 and 2024
Operating Expense
The following table sets forth the Company’s selected statements of operations data for the following periods:
|
Three Months Ended |
||||||||||||
|
2025 |
2024 |
Change |
||||||||||
|
Operating expenses |
|
|
|
|
|
|
||||||
|
Research and development expenses |
$ |
619,693 |
|
$ |
— |
|
$ |
619,693 |
|
|||
|
General and administrative expenses |
|
1,224,546 |
|
|
299,999 |
|
|
924,547 |
|
|||
|
Loss from operations |
|
(1,844,239 |
) |
|
(299,999 |
) |
|
(1,544,240 |
) |
|||
|
Other expenses |
|
|
|
|
|
|
||||||
|
Interest income |
|
56,426 |
|
|
116 |
|
|
56,310 |
|
|||
|
Change in fair value of warrant liability |
|
(12,859 |
) |
|
— |
|
|
(12,859 |
) |
|||
|
Interest expense |
|
(6,301 |
) |
|
(32,638 |
) |
|
26,337 |
|
|||
|
Net loss |
$ |
(1,781,255 |
) |
$ |
(332,521 |
) |
$ |
(1,448,734 |
) |
|||
Revenues
For the three months ended September 30, 2025 and 2024, the Company had no revenue.
35
Operating expenses
Research and development expense
The following table summarizes the year-over-year changes in research and development expenses for the three months ended September 30, 2025:
|
Three Months Ended |
|||||||||
|
2025 |
2024 |
Change |
|||||||
|
Payroll expenses |
$ |
8,871 |
$ |
— |
$ |
8,871 |
|||
|
Clinical trials |
|
557,430 |
|
— |
|
557,430 |
|||
|
Compensation – stock and stock options |
|
13,629 |
|
— |
|
13,629 |
|||
|
Other |
|
39,763 |
|
— |
|
39,763 |
|||
|
$ |
619,693 |
$ |
— |
$ |
619,693 |
||||
Research and development expenses totaled $619,693 for the three months ended September 30, 2025, compared to no such expenses for the same period in 2024, reflecting an increase of $619,693. This increase was primarily driven by the availability of funding, which supported higher overall research and development spending. The increase was mainly attributable to stock-based compensation of approximately $14,000, clinical trial costs of approximately $557,000, and other research and development expenses totaling approximately $40,000.
General and administrative expenses
The following table summarizes the year-over-year changes in general and administrative expenses for the three months ended September 30, 2025:
|
Three Months Ended |
|||||||||
|
2025 |
2024 |
Change |
|||||||
|
Payroll expenses |
$ |
384,451 |
$ |
91,000 |
$ |
293,451 |
|||
|
Professional services |
|
549,590 |
|
202,995 |
|
346,595 |
|||
|
Compensation – stock and stock options |
|
29,045 |
|
— |
|
29,045 |
|||
|
Insurance |
|
54,255 |
|
— |
|
54,255 |
|||
|
Office expenses |
|
106,131 |
|
3,818 |
|
102,313 |
|||
|
Other general and administrative |
|
101,074 |
|
2,186 |
|
98,888 |
|||
|
$ |
1,224,546 |
$ |
299,999 |
$ |
924,547 |
||||
General and administrative expenses totaled $1,224,546 for the three months ended September 30, 2025, compared to $299,999 for the same period in 2024, representing an increase of $924,547. The increase was primarily driven by higher stock compensation costs and expanded operational activities. Specifically, the change included an increase in professional services of approximately $347,000, stock-based compensation of approximately $29,000, insurance expenses of approximately $54,000, office expenses of approximately $102,000, and other general and administrative costs of approximately $99,000.
Other Income (expense)
The following table summarizes the year-over-year changes in other income (expense) for the periods presented:
|
Three Months Ended |
|||||||||||
|
2025 |
2024 |
Change |
|||||||||
|
Interest income |
$ |
56,426 |
|
$ |
116 |
|
$ |
56,310 |
|||
|
Change in fair value of warrant liability |
|
12,859 |
|
|
— |
|
|
12,859 |
|||
|
Interest expense |
|
(6,301 |
) |
|
(32,638 |
) |
|
26,337 |
|||
|
$ |
62,984 |
|
$ |
(32,522 |
) |
$ |
95,506 |
||||
36
Other income was $62,984 for the three months ended September 30, 2025, compared to other expense of $32,522 for the same period in 2024, representing an increase in income of $95,506. The increase was mainly due to an increase in interest income of approximately $56,000 and a decrease in interest expense of approximately $26,000.
Net Loss
Net loss was $1,781,255 for the three months ended September 30, 2025, compared to net loss of $332,521 in the same period of 2024, representing an increased loss of $1,448,734. The increase was mainly due to the increase in both general and administrative expenses and research and development expenses due to higher payroll expenses, professional services and expanded operational and research and development activities.
Results of operations for the nine months ended September 30, 2025 and 2024
Operating Expense
The following table sets forth the Company’s selected statements of operations data for the following periods:
|
Nine Months Ended |
||||||||||||
|
2025 |
2024 |
Change |
||||||||||
|
Operating expenses |
|
|
|
|
|
|
||||||
|
Research and development expenses |
$ |
1,271,477 |
|
$ |
— |
|
$ |
1,271,477 |
|
|||
|
General and administrative expenses |
|
3,601,034 |
|
|
999,482 |
|
|
2,601,552 |
|
|||
|
Loss from operations |
|
(4,872,511 |
) |
|
(999,482 |
) |
|
(3,873,029 |
) |
|||
|
Other expenses |
|
|
|
|
|
|
||||||
|
Interest income |
|
71,676 |
|
|
2,794 |
|
|
68,882 |
|
|||
|
Change in fair value of warrant liability |
|
22,377 |
|
|
— |
|
|
22,377 |
|
|||
|
Interest expense |
|
(67,387 |
) |
|
(81,669 |
) |
|
14,282 |
|
|||
|
Net loss |
$ |
(4,845,845 |
) |
$ |
(1,078,357 |
) |
$ |
(3,767,488 |
) |
|||
Revenues
For the nine months ended September 30, 2025 and 2024, the Company had no revenue.
Operating expenses
Research and development expenses
The following table summarizes the year-over-year changes in research and development expenses for the nine months ended September 30, 2025:
|
Nine Months Ended |
|||||||||
|
2025 |
2024 |
Change |
|||||||
|
Payroll expenses |
$ |
81,027 |
$ |
— |
$ |
81,027 |
|||
|
Clinical trials |
|
674,967 |
|
— |
|
674,967 |
|||
|
Compensation – stock and stock options |
|
452,258 |
|
— |
|
452,258 |
|||
|
Other |
|
63,225 |
|
— |
|
63,225 |
|||
|
Total research and development expenses |
$ |
1,271,477 |
$ |
— |
$ |
1,271,477 |
|||
Research and development expenses totaled $1,271,477 for the nine months ended September 30, 2025, compared to no such expenses for the same period in 2024, reflecting an increase of $1,271,477. This increase was primarily driven by the availability of funding, which supported higher overall research and development spending. The increase was mainly attributable to payroll expenses of approximately $81,000, stock-based compensation of approximately $452,000, clinical trial costs of approximately $675,000, and other research and development expenses totaling approximately $63,000.
37
General and administrative expenses
The following table summarizes the year-over-year changes in general and administrative expenses for the nine months ended September 30, 2025:
|
Nine Months Ended |
|||||||||
|
2025 |
2024 |
Change |
|||||||
|
Payroll expenses |
$ |
594,929 |
$ |
297,000 |
$ |
297,929 |
|||
|
Professional services |
|
1,138,448 |
|
669,923 |
|
468,525 |
|||
|
Compensation – stock and stock options |
|
1,482,469 |
|
— |
|
1,482,469 |
|||
|
Insurance |
|
85,510 |
|
— |
|
85,510 |
|||
|
Office expenses |
|
176,508 |
|
14,144 |
|
162,364 |
|||
|
Other general and administrative |
|
123,170 |
|
18,415 |
|
104,755 |
|||
|
Total general and administrative expenses |
$ |
3,601,034 |
$ |
999,482 |
$ |
2,601,552 |
|||
General and administrative expenses totaled $3,601,034 for the nine months ended September 30, 2025, compared to $999,482 for the same period in 2024, representing an increase of $2,601,552. The increase was primarily driven by higher stock compensation costs and expanded operational activities. Specifically, the change included the increases in professional services of approximately $469,000, stock-based compensation of approximately $1,482,000, payroll expenses of approximately $298,000, insurance expenses of approximately $86,000 and office expenses of approximately $162,000.
Other Expense
The following table summarizes the year-over-year changes in other expenses for the periods presented:
|
Nine Months Ended |
|||||||||||
|
2025 |
2024 |
Change |
|||||||||
|
Interest income |
$ |
71,676 |
|
$ |
2,794 |
|
$ |
68,882 |
|||
|
Change in fair value of warrant liability |
|
22,377 |
|
|
— |
|
|
22,377 |
|||
|
Interest expense |
|
(67,387 |
) |
|
(81,669 |
) |
|
14,282 |
|||
|
$ |
26,666 |
|
$ |
(78,875 |
) |
$ |
105,541 |
||||
Other income was $26,666 for the nine months ended September 30, 2025, compared to other expense of $78,875 for the same period in 2024, representing an increase in other income of $105,541. The increase was mainly due to an increase in interest income of approximately $69,000 corresponding with the decrease in interest expense of approximately $14,000 due to conversion of the notes, as well as gain as a result of the change in fair value of warrant liability for approximately $22,000.
Net Loss
Net loss was $4,845,845 for the nine months ended September 30, 2025, compared to net loss of $1,078,357 in the same period of 2024, representing an increase in loss of $3,767,488. The increase was mainly due to the increase in both general and administrative expenses and research and development expenses due to higher stock compensation costs and expanded operational and research and development activities.
Liquidity and Capital Resources
The Company has generated no revenue, has incurred operating losses since inception, expects to continue to incur significant operating losses for the foreseeable future and may never become profitable. Until such time as the Company is able to establish a revenue stream, it is dependent upon obtaining necessary equity and/or debt financing to continue operations. The Company cannot make any assurances that sales will commence in the near term or that additional financing will be available to it on acceptable terms or at all. This could negatively impact our business and operations and could also lead to the reduction of our operations.
38
Cash Flows
The following table presents selected financial information and statistics for each of the periods shown below:
|
Nine Months Ended |
||||||||||||
|
2025 |
2024 |
Change |
||||||||||
|
Net cash used in operating activities |
$ |
(5,107,575 |
) |
$ |
(633,910 |
) |
$ |
(4,473,665 |
) |
|||
|
Net cash used in investing activities |
|
(35,909 |
) |
|
— |
|
|
(35,909 |
) |
|||
|
Net cash provided by financing activities |
|
12,126,646 |
|
|
250,000 |
|
|
11,876,646 |
|
|||
|
Net increase (decrease) in cash |
$ |
6,983,162 |
|
$ |
(383,910 |
) |
$ |
7,367,072 |
|
|||
During the nine months ended September 30, 2025, operating activities used approximately $5,108,000 of cash, primarily resulting from a net loss of $4,859,332, partially offset by non-cash stock-based compensation for stock and stock options grants in the approximate amount of $1,700,000 and $235,000, respectively, accretion expense of approximately $40,000, and changes in operating assets and liabilities of approximate decrease of $2,243,000, mainly due to increase in prepaid research costs and prepaid insurance and decrease in accounts payable and accrued expenses.
During the nine months ended September 30, 2025, operating activities used approximately $634,000 of cash, primarily resulting from a net loss of $1,078,357, partially offset by non-cash interest expense-related parties of approximately $27,000, accretion expense of approximately $55,000, and positive changes in operating assets and liabilities of approximately $363,000.
Investing activities
During the nine months ended September 30, 2025 and 2024 investing activities used approximately $36,000 and $0, respectively, resulting from acquired furniture and equipment.
Financing activities
During the nine months ended September 30, 2025, financing activities provided approximately $12,126,600 of cash. This was primarily attributable to net proceeds from the issuance of common stock in the IPO of $11,953,046, proceeds from notes payable from related parties of $250,000, and cash advances from related parties of $17,400, partially offset by cash advances paid to related parties in the amount of $93,800.
During the nine months ended September 30, 2024, financing activities provided $250,000 of cash, consisting entirely of proceeds from notes payable from related parties.
Contractual Obligations and Commitments
See Note 6 — Debt, and Note 8 — Commitments and Contingencies, of the notes to the Company’s financial statements as of and for the three months ended September 30, 2025 included elsewhere in this Quarterly Report for further discussion of the Company’s commitments and contingencies.
Off-Balance Sheet Arrangements
The Company is not party to any off-balance sheet transactions. The Company has no guarantees or obligations other than those which arise out of normal business operations.
Critical Accounting Policies and Significant Judgments and Estimates
The Company’s management’s discussion and analysis of its financial condition and results of operations is based on its financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these unaudited condensed financial statements requires Apimeds Pharmaceuticals US, Inc. to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities as of the date of the balance sheet and the reported amounts of expenses during the reporting period. In accordance with U.S. GAAP, Apimeds Pharmaceuticals US, Inc. evaluates its estimates and judgments on an ongoing basis. The most significant estimates relate to convertible instruments. Apimeds Pharmaceuticals US, Inc. bases its estimates and
39
assumptions on current facts, historical experiences, and various other factors that Apimeds Pharmaceuticals US, Inc. believes are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
The Company defines its critical accounting policies as those accounting principles that require it to make subjective estimates and judgments about matters that are uncertain and are likely to have a material impact on its financial condition and results of operations, as well as the specific manner in which the Company applies those principles. While its significant accounting policies are more fully described in Note 2 to its financial statements, the Company believes the following are the critical accounting policies used in the preparation of its unaudited condensed financial statements that require significant estimates and judgments.
Convertible Instruments
The Company evaluates and accounts for conversion options embedded in convertible instruments in accordance with ASC 815 “Derivatives and Hedging Activities”.
The Company accounts for convertible instruments (when we have determined that the embedded conversion options should not be bifurcated from their host instruments) as follows: The Company records when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are amortized over the term of the related debt to their stated date of redemption.
40
INFORMATION ABOUT THE BUSINESS OF MINDWAVE INNOVATIONS INC
Unless the context otherwise indicates or requires, all references in this section to “we,” “us,” “our,” “our company” “the Company” and “MindWave” refer to MindWave Innovations Inc
MindWave Innovations Inc (“MindWave,” the “Company,” “we,” “us” or “our”) is a technology platform company focused on institutional Digital Asset Treasury (“DAT”) solutions, centered on enabling corporations and institutions to hold, manage, and generate yield on Bitcoin reserves through a compliant, scalable infrastructure. Our strategic model integrates secure digital treasury wallets, AI-supported Bitcoin yield programs, and a validator-enabled ecosystem supported by our native token, $NILA. TechyTrade Innovations Pte. Ltd. is a Singapore exempt private company and wholly owned subsidiary of MindWave Innovations Inc (“TechyTrade (Singapore)”). TechyTrade FZ LLC is a limited liability company operating under the laws of the Ras Al Khaimah Economic Zone is a wholly owned subsidiary of TechyTrade Singapore (“TechyTrade (Dubai)”). MindWave operates through an international structure that includes TechyTrade (Singapore) and TechyTrade (Dubai), which contains the primary business operations of MindWave.
Our balance sheet strategy is designed to align with our DAT offering. TechyTrade (Dubai) owns or controls 1,000 Bitcoin free of encumbrances, identifiable and segregated within a sub-wallet architecture created and administered by MindWave Ltd. for the benefit of TechyTrade (Dubai), with private keys and beneficial ownership retained by TechyTrade (Dubai). TechyTrade (Dubai) has no indebtedness other than trade payables, which were less than fifty percent of cash on hand. This reserve posture is intended to preserve purchasing power, provide continuous exposure to Bitcoin, and support our yield infrastructure without requiring asset sales.
We intend to commercialize our principal DAT offerings for institutional clients, which comprise: (i) secure corporate Bitcoin treasury infrastructure designed for public-company balance sheets and institutional controls; (ii) AI-supported Bitcoin yield strategies intended to deliver risk-managed, programmatic yield including InsureTech; and (iii) a validator-enabled ecosystem that supports utility and governance via $NILA across interoperable verticals, including AdTech engagement platforms and ClimateTech impact systems, as described in our technical materials. Custody of client assets is expected to occur through regulated third-party providers and institutional-grade wallet solutions, implemented within a segregation and control framework consistent with institutional compliance requirements.
Our operating framework is designed around Bitcoin as the core reserve and risk collateral reference for treasury and yield solutions. We expect to avoid hybrid collateral models in favor of a Bitcoin-centric approach that is transparent, simple, and consistent with our treasury and platform design. Within our ecosystem, $NILA functions as an economic and governance substrate to activate services, enable staking mechanisms that align incentives, and facilitate validator economics across our interoperable platforms.
We intend to serve corporate treasuries and institutions that maintain or plan to maintain Bitcoin reserves and require scalable, compliant DAT capabilities. We also expect to support high-net-worth individuals and funds seeking institutional controls for Bitcoin reserve management and programmatic yield. Consistent with institutional practices, our anticipated operations emphasize transparency and risk management, including conservative collateralization where relevant to client programs, real-time risk monitoring, and robust anti-money laundering (AML) and know-your-customer (KYC) processes. We are pursuing strategic relationships with financial institutions, placement agents, and digital-asset service providers to support custody, treasury operations, and capital formation for expansion of our platform.
We believe that our combination of (i) an institutional Bitcoin reserve posture supported by segregated custody architecture and independent attestation, (ii) AI-enhanced yield strategies integrated into a controlled DAT stack, and (iii) a validator-enabled ecosystem powered by $NILA positions us to meet emerging institutional demand for Bitcoin treasury solutions. In connection with our merger with Apimeds Pharmaceuticals US, Inc., our DAT platform is expected to be operated within a publicly listed structure, with Apimeds’ biopharmaceutical business continuing within a subsidiary post-closing, and capital formation initiatives contemplated to support both the DAT platform and biopharmaceutical development. We believe this structure enhances our ability to scale client adoption while maintaining regulatory discipline and institutional-grade controls.
41
General Development of Our Business
MindWave Innovations Inc was incorporated in the State of Delaware in 2025. On December 1, 2025, MindWave signed and closed an Agreement and Plan of Merger with Apimeds Pharmaceuticals US, Inc. (“Apimeds”), Apimeds Merger Sub, Inc. (“Merger Sub”), Lokahi Therapeutics, Inc., and Erik Emerson, pursuant to which Merger Sub merged with and into MindWave, with MindWave surviving as a wholly owned subsidiary of Apimeds (the “Merger”).
Following the Merger, we have focused our development efforts on scaling an institutional DAT platform. Consistent with our treasury reserve posture, our group maintains Bitcoin as its core reserve asset. As previously mentioned, TechyTrade (Dubai) owns and controls 1,000 Bitcoin, identifiable and segregated in sub-wallets administered by MindWave Ltd.
Our principal activities consist of: (i) building our institutional DAT stack, including secure corporate Bitcoin treasury infrastructure and AI-supported Bitcoin yield strategies; (ii) implementing and attesting to our Bitcoin reserve and segregated custody architecture; and (iii) forming relationships with regulated custodians, wallet and validator providers, financial institutions, and placement agents to support custody, risk management, compliance (including AML/KYC), and capital formation. We are in the execution stage and are preparing for commercialization of our DAT offerings.
Roadmap and Milestones
Our near-term priorities focus on: (i) onboarding initial institutional clients to our corporate Bitcoin treasury infrastructure; (ii) progressing AI-supported, risk-managed yield programs under defined policy and control frameworks; (iii) standing up enterprise validator services to support network participation and governance; and (iv) advancing development of our ClimateTech, AdTech, and InsurTech verticals to interoperate with our DAT stack. Over time, we intend to expand treasury management capabilities, scale validator operations, and develop ecosystem utility, subject to applicable regulatory requirements, market conditions, and governance approvals.
Our Treasury Strategy and Digital Asset Treasury Platform
Bitcoin Treasury Strategy
We believe that Bitcoin is an attractive reserve asset because (i) it can serve as a store of value supported by a robust, public, open-source architecture that is independent of sovereign monetary policy, (ii) its fixed supply offers the potential to serve as a long-term hedge against inflation and, as adoption increases, the opportunity for appreciation, and (iii) the Bitcoin network provides infrastructure for financial and technological innovation.
We were formed with the intention of operating an institutional-focused Digital Asset Treasury (“DAT”) platform, and our group has adopted a Bitcoin-centric reserve posture aligned with that platform. Under this posture, our treasury reserve assets consist principally of:
• Cash and cash equivalents sufficient for working capital and operational needs; and
• Bitcoin held at the operating-subsidiary level as our primary reserve asset, maintained within a segregated sub-wallet architecture and free of encumbrances, subject to business needs and market conditions.
Consistent with this reserve posture, we may from time to time evaluate capital raising transactions to support platform development, working capital, and expansion of our reserve strategy. We expect future capital allocation decisions to consider market conditions, risk management, regulatory considerations, and anticipated operating requirements. Our strategy contemplates that we may (i) periodically rebalance or sell Bitcoin for general corporate purposes, (ii) utilize our Bitcoin reserve within conservative, risk-managed programs that support our yield and validator infrastructure, and (iii) evaluate compliant structures to generate programmatic returns consistent with institutional practices and applicable law.
We also plan to conduct advocacy and educational initiatives regarding institutional Bitcoin treasury standards, custody segregation, controls, and reporting, including thought leadership and partnerships intended to support the broader adoption of compliant corporate Bitcoin treasury operations.
42
DAT Yield and Validator Infrastructure
Our core offerings center on an institutional DAT stack designed to help corporations and institutions hold, manage, and generate yield on Bitcoin reserves. Key components include:
• Institutional treasury infrastructure. Secure wallet architecture with segregation and controls designed to align with public-company balance sheet requirements and institutional compliance frameworks.
• AI-supported yield programs. Risk-managed strategies intended to produce programmatic returns on Bitcoin reserves, with governance and monitoring controls suitable for institutional participants.
• Validator-enabled ecosystem. Operations that support network economics and governance across our ecosystem, including utility powered by our native token, $NILA.
Client assets, if and when onboarded, are expected to be held through regulated third-party custodians or institutional-grade wallet solutions within a segregation and control framework consistent with AML/KYC and other applicable requirements. We emphasize conservative collateralization (where relevant to client programs), real-time risk monitoring, and robust compliance processes.
Our Bitcoin Holdings
On March 31, 2025, TechyTrade (Dubai) entered into an Asset Purchase Agreement with MindWave Ltd., pursuant to which TechyTrade (Dubai) acquired 1,000 bitcoin. The bitcoin are held free of encumbrances within a segregated sub-wallet architecture administered by MindWave Ltd. We did not sell any bitcoin during 2024 or 2025.
Our Industry Overview
Bitcoin Industry
Bitcoin is a digital asset that is issued by and transmitted through an open-source protocol, known as the Bitcoin protocol, collectively maintained by a peer-to-peer network of decentralized user nodes. This network hosts a public transaction ledger, known as the Bitcoin blockchain, on which bitcoin holdings and all validated transactions that have ever taken place on the Bitcoin network are recorded. Balances of bitcoin are stored in individual “wallet” functions, which associate network public addresses with one or more “private keys” that control the transfer of bitcoin. The Bitcoin blockchain can be updated without any single entity owning or operating the network.
Creation of New Bitcoin and Limits on Supply
The Bitcoin protocol limits the total number of bitcoins that can be generated over time to 21 million. As of [—], 2025, approximately 19.93 million bitcoins have been generated. New bitcoins are created and allocated by the Bitcoin protocol through a “mining” process that rewards users that validate transactions in the Bitcoin blockchain. Validated transactions are added in “blocks” approximately every 10 minutes. The mining process serves to validate transactions and secure the Bitcoin network. Mining is a competitive and costly operation that requires a large amount of computational power to solve complex mathematical algorithms. This expenditure of computing power is known as “proof of work.”
To incentivize miners to incur the costs of mining bitcoin, the Bitcoin protocol rewards miners that successfully validate a block of transactions with newly generated bitcoin. The current reward for miners that successfully validate a block of transactions is 3.125 bitcoin per mined block. The mining reward is reduced by half, which is referred to as a bitcoin halving, after every 210,000 blocks are mined. This has historically occurred approximately every four years. The most recent bitcoin halving occurred in April 2024, and the next bitcoin halving is expected to occur sometime in 2028.
Modifications to the Bitcoin Protocol
Bitcoin is an open-source network that has no central authority, so no one person can unilaterally make changes to the software that runs the network. However, there is a core group of developers that maintains the code for the Bitcoin protocol, and they can propose changes to the source code and release periodic updates and other changes. Unlike most software that has a central entity that can push updates to users, bitcoin is a peer-to-peer network in which individual
43
network participants, called nodes, decide whether to upgrade the software and accept the new changes. As a practical matter, a modification becomes part of the Bitcoin protocol only if the proposed changes are accepted by participants collectively having more than 50% of the processing power, known as hash rate, on the network. If a certain percentage of the nodes reject the changes, then a “fork” takes place, and participants can choose the version of the software they want to run.
Forms of Attack Against the Bitcoin Network and Wallets
Blockchain technology has many built-in security features that make it difficult for hackers and other malicious actors to corrupt the protocol or blockchain. However, as with any computer network, the Bitcoin network may be subject to certain attacks. Some forms of attack include unauthorized access to wallets that hold bitcoin and direct attacks, like “51% attacks” or “denial-of-service attacks” on the Bitcoin network.
Bitcoin is controllable only by the possessor of both the unique public key and private key(s) relating to the local or online digital wallet in which the bitcoin is held. Private keys used to access bitcoin balances are not widely distributed and are typically held on hardware (which can be physically controlled by the holder or by a third party such as a custodian) or via software programs on third-party servers. One form of obtaining unauthorized access to a wallet occurs following a phishing attack where the attacker deceives the victim and manipulates them into sharing their private keys for their digital wallet or other sensitive information. Other similar attacks may also result in the loss of private keys and the inability to access, and effective loss of, the corresponding bitcoin.
A “51% attack” may occur when a group of miners attain more than 50% of the Bitcoin network’s mining power, thereby enabling them to control the Bitcoin network and protocol and manipulate the blockchain. A “denial-of-service attack” occurs when legitimate users are unable to access information systems, devices, or other network resources due to the actions of a malicious actor flooding the network with traffic until the network is unable to respond or crashes. The Bitcoin network has been, and can be in the future, subject to denial-of-service attacks, which can result in temporary delays in block creation and in the transfer of bitcoin.
Bitcoin Industry Participants
The primary Bitcoin industry participants are miners, investors and traders, digital asset exchanges and service providers, including custodians, brokers, payment processors, wallet providers and financial institutions.
Miners. Miners range from bitcoin enthusiasts to professional mining operations that design and build dedicated mining machines and data centers, including mining pools, which are groups of miners that act cohesively and combine their processing power to mine bitcoin blocks. See “— Creation of New Bitcoin and Limits on Supply” above.
Investors and Traders. Bitcoin investors and traders include individuals and institutional investors who, directly or indirectly, purchase, hold, and sell bitcoin or bitcoin-based derivatives. On January 10, 2024, the Securities and Exchange Commission (“SEC”) issued an order approving several applications for the listing and trading of shares of spot bitcoin exchange-traded products (“ETPs”) on U.S. national securities exchanges. While the SEC had previously approved exchange-traded funds where the underlying assets were bitcoin futures contracts, this order represented the first time the SEC approved the listing and trading of ETPs that acquire, hold and sell bitcoin directly. ETPs can be bought and sold on a stock exchange like traditional stocks, and provide investors with another means of gaining economic exposure to bitcoin through traditional brokerage accounts.
Digital Asset Exchanges. Digital asset exchanges provide trading venues for purchases and sales of bitcoin in exchange for fiat or other digital assets. Bitcoin can be exchanged for fiat currencies, such as the U.S. dollar, at rates of exchange determined by market forces on bitcoin trading platforms, which are not regulated in the same manner as traditional securities exchanges. In addition to these platforms, over-the-counter markets and derivatives markets for bitcoin also exist. The value of bitcoin within the market is determined, in part, by the supply of and demand for bitcoin in the global bitcoin market, market expectations for the adoption of bitcoin as a store of value, the number of merchants that accept bitcoin as a form of payment, and the volume of peer-to-peer transactions, among other factors.
Service providers. Service providers offer a multitude of services to other participants in the Bitcoin industry, including custodial and trade execution services, commercial and retail payment processing, wallet solutions and financial institutions. If adoption of the Bitcoin network continues to materially increase, we anticipate that service providers may expand the currently available range of services and that additional parties will enter the service sector for the Bitcoin network.
44
Other Digital Assets
As of the date of this Information Statement, bitcoin was the largest digital asset by market capitalization. However, numerous alternative digital assets exist, and many entities, including consortia and financial institutions, are actively researching and investing resources in blockchain platforms and digital assets that utilize consensus mechanisms other than proof-of-work mining, which is employed by the Bitcoin network. For example, in late 2022, the Ethereum network transitioned to a “proof-of-stake” mechanism for validating transactions that requires significantly less computing power than proof-of-work mining. Other alternative digital assets that compete with bitcoin in certain ways include “stablecoins,” which are designed to maintain a constant price because of their issuers’ promise to hold high-quality liquid assets (such as U.S. dollar deposits and short-term U.S. treasury securities) equal to the total value of stablecoins in circulation. Stablecoins have grown rapidly as an alternative to bitcoin and other digital assets as a medium of exchange and store of value, particularly on digital asset trading platforms. As of December 31, 2024, two of the eight largest digital assets by market capitalization were U.S. dollar-backed stablecoins.
Additionally, central banks in some countries have started to introduce digital forms of legal tender. For example, China’s central bank digital currency (“CBDC”) project was made available to consumers in January 2022, and governments including the United States and the European Union have discussed the potential creation of new CBDCs.
Our Market Opportunity for Institutional Bitcoin Treasury and DAT Solutions
The use of Bitcoin as a corporate treasury reserve asset has expanded as companies and institutions evaluate alternatives to traditional cash management strategies. While fiat currency and short-term government securities remain predominant, the emergence of U.S. spot Bitcoin exchange-traded funds and increasing recognition of Bitcoin as an investable asset class have contributed to broader institutional acceptance. A growing number of public and private companies, investment funds, and other institutional allocators have begun to evaluate or adopt Bitcoin reserves as a potential long-term store of value and as a component of diversified treasury strategies.
Despite these developments, adoption of Bitcoin for treasury purposes remains limited relative to the overall size of global corporate treasury balances. We believe this gap highlights a meaningful market opportunity for institutional-grade platforms that can provide compliant, transparent, and risk-managed infrastructure for Bitcoin treasury operations. Key needs we see in the market include secure custody with segregation and controls; governance frameworks aligned with public-company standards; accounting and reporting support; risk-managed yield programs; and integration into existing treasury workflows.
We believe there is an opportunity to differentiate by offering an integrated DAT solution that combines: (i) corporate Bitcoin treasury infrastructure designed for institutional policies and controls; (ii) AI-supported, risk-managed yield programs intended to produce programmatic returns on reserve assets; and (iii) a validator-enabled ecosystem that supports network participation and governance, with utility powered by our native token, $NILA. As institutional comfort and regulatory clarity continue to develop, we expect the use of Bitcoin in treasury management to expand, supported by demand for transparent, regulated, and operationally disciplined solutions.
In our view, market growth will be driven by several secular trends: increased institutional access to Bitcoin through regulated vehicles and service providers; enhancements in custody, compliance, and accounting standards; and the need for end-to-end platforms that allow corporate treasury teams to implement Bitcoin strategies without building bespoke infrastructure. Our platform is being developed to address these requirements by providing segregation, controls, monitoring, and reporting consistent with institutional expectations, along with programmatic yield and validator capabilities designed to complement a Bitcoin-centric reserve posture.
Our Products and Services
Our principal planned products and services focus on delivering an institutional Digital Asset Treasury (“DAT”) platform that enables corporations and institutions to hold, manage, and generate yield on Bitcoin reserves through a compliant, scalable infrastructure. We are in the development execution stage and are actively building the underlying architecture, governance, and partnerships necessary to support future commercialization.
45
Institutional DAT Platform
• Corporate Bitcoin treasury infrastructure. Segregated wallet architecture, permissions and controls, and reporting designed to align with institutional compliance frameworks and public-company balance sheet requirements.
• AI-supported yield programs. Risk-managed strategies intended to produce programmatic returns on Bitcoin reserves, with governance, monitoring, and risk limits tailored for institutional participants.
• Validator-enabled ecosystem. Network operations that support validator economics and governance across our interoperable platforms, with utility powered by our native token, $NILA.
• Compliance and risk management. Policies and tooling addressing AML/KYC, ongoing monitoring, collateral and reserve discipline where relevant to client programs, and real-time risk analytics.
• Advisory and enablement. Onboarding and operational support to help clients adopt institutional Bitcoin treasury standards, including segregation, reconciliation, and controls.
Treasury Reserve and Bitcoin Strategy
Our group maintains a Bitcoin-centric reserve posture that aligns with our DAT platform. This approach is intended to preserve purchasing power, provide continuous exposure to Bitcoin, and support our yield and validator infrastructure. From time to time, we may evaluate capital raising transactions to fund platform development, operations, and reserve strategy, subject to market conditions, risk management, regulatory considerations, and anticipated operating needs.
Custody and Execution Services
We intend to utilize regulated third-party custodians and institutional-grade wallet solutions for the safekeeping of any client assets onboarded to our platform, as well as our operating-subsidiary reserves. These providers are expected to support secure custody, segregation, and controls, and to offer affiliated execution services for Bitcoin acquisitions and dispositions consistent with institutional best practices.
Ecosystem Verticals Powered by $NILA
In addition to our core Digital Asset Treasury (“DAT”) platform, we operate interoperable verticals powered by our native token, $NILA, which enables governance, utility, and value flow across our ecosystem.
• ClimateTech (AQUAE Impact). One our ecosystems is a blockchain-powered sustainability engine intended to generate verifiable, insured environmental impact outcomes, including fractionalized credits linked to analog forest projects and other conservation efforts. The roadmap contemplates phases focused on expanding analog forest capacity, introducing water-conservation and biodiversity credits, and building a secondary market for the exchange of such credits.
• AdTech (Wave+). Another ecosystem is a micro-engagement platform to convert user attention into tokenized value for brand and mission-aligned campaigns. The platform is designed to support daily active engagement, with contemplated revenue streams from ad placements, partner campaigns, and conversion fees.
• InsurTech (Institutional Insurance Engine). We are designing an insurance framework to complement digital treasury strategies through surplus-capital-style models backed by strengthened, Bitcoin-centric balance sheets. The goal is to support treasury protection, parametric digital-asset coverage, and institutional insurance primitives that can be integrated with our DAT platform.
Validator Infrastructure
We intend to operate enterprise-grade validator nodes to support network participation, governance, and rewards within our ecosystem and for institutional clients. The validator stack is being developed with an emphasis on high availability, slashing prevention, hardware security (including HSM/TEE-based protections), governance controls, and policy-limited MEV/PBS participation. Anticipated revenue drivers include staking rewards, protocol incentives, enterprise validator service fees, and ecosystem transaction fees.
46
Revenue Model
Our contemplated revenue model includes multiple streams that align with institutional treasury adoption:
• Treasury Management Fees: Asset-under-management-based fees for corporate treasury wallets and related services.
• Performance and Program Fees: Participation in net returns from AI-supported yield programs, subject to institutional governance and risk policies.
• Validator Services and Protocol Incentives: Enterprise staking and validator service fees, protocol-derived incentives, and transaction-based revenues tied to ecosystem activity. Monetization associated with ClimateTech (e.g., fractionalized credits), AdTech (campaign and placement revenue), and InsurTech-related structures, where applicable.
Our Customers
We target enterprise clients that require institutional governance, custody segregation, and audit-ready reporting for Bitcoin treasury operations, including public and private corporate treasury teams, institutional investors and asset managers, financial institutions and intermediaries, and high-net-worth organizations with significant Bitcoin reserves. We aim to integrate into CFO, treasury, and risk committee workflows, including policy design, compliance and accounting support, and board-level reporting.
We have generated revenues and do not currently rely on any single customer for more than 10% of our revenues.
Our Market Positioning
We believe our market positioning is strengthened by the integration of our institutional Digital Asset Treasury (“DAT”) platform, segregated custody architecture, and Bitcoin-centric reserve posture. Our platform is designed to enable corporations and institutions to implement Bitcoin treasury operations with the governance, controls, and reporting expected by institutional stakeholders, while complementing those reserves with AI-supported, risk-managed yield programs and validator participation within our broader ecosystem.
Our Bitcoin holdings are maintained primarily as a treasury reserve asset intended to support our balance sheet and operational resilience. Our reserve posture reinforces our credibility with institutional clients by aligning our incentives with a Bitcoin-centric treasury strategy and by demonstrating disciplined custody, segregation, and control practices.
By maintaining a Bitcoin-centric reserve posture and building end-to-end DAT infrastructure, we aim to provide a comprehensive, compliant, and scalable pathway for institutions to adopt Bitcoin in treasury management. This integration of reserve management, custody controls, yield programs, and validator operations is intended to enhance risk management, support operational discipline, and provide a foundation for long-term value creation.
Our Competitive Strengths
Platform Governance and $NILA Utility
$NILA functions as a governance and utility token within our ecosystem, facilitating validator participation, program access, and value exchange across verticals. Governance processes are designed to be policy-driven and auditable, with the objective of aligning incentives among stakeholders while maintaining institutional controls. Within our validator framework, we intend to limit protocol participation to policy-permitted activities and to maintain risk controls, including stake diversification, slashing protection, and real-time monitoring.
We believe the following strengths position us to compete effectively in the emerging institutional Bitcoin treasury market:
• Institutional DAT stack. End-to-end infrastructure for corporate Bitcoin treasury operations, including secure wallet architecture, permissions and controls, reconciliation, and reporting aligned with institutional requirements.
47
• Segregated custody architecture. Use of institutional-grade wallet solutions and regulated third-party providers for safekeeping, with segregation and control frameworks designed to mitigate counterparty and operational risks.
• Bitcoin-centric reserve posture. A treasury strategy centered on Bitcoin reserves intended to preserve purchasing power, maintain continuous exposure, and support platform credibility with institutional clients.
• AI-supported yield programs. Risk-managed strategies intended to produce programmatic returns on reserve assets, governed by policies, monitoring, and limits consistent with institutional risk management.
• Validator-enabled ecosystem. Network participation that supports validator economics and governance across interoperable platforms, with utility powered by our native token, $NILA.
• Compliance and governance. Emphasis on AML/KYC, surveillance, and real-time risk monitoring, together with operational controls designed to align with institutional and public-company standards.
• Scalable partner network. Strategic relationships with custodians, wallet providers, financial institutions, and other service partners to support onboarding, execution, and operational scale.
• Operational discipline. A focus on segregation, transparency, and audit-ready processes to support institutional adoption and long-term sustainability.
We believe our integrated approach — combining corporate Bitcoin treasury infrastructure, AI-supported yield programs, validator operations, and interoperable verticals powered by $NILA — addresses key adoption barriers by offering a policy-driven, audit-ready, and scalable pathway for institutions. Our multi-vertical design is intended to enhance network effects and create diversified revenue opportunities aligned with institutional compliance and governance expectations.
Custody of Our Bitcoin
Our Bitcoin reserve is maintained within a segregated sub-wallet architecture created and administered by MindWave Ltd. for the benefit of our operating subsidiary, TechyTrade (Dubai). TechyTrade (Dubai) holds the private keys and retains full beneficial ownership and control of the segregated wallet(s). The 1,000 Bitcoin are identifiable, segregated, and held free and clear of any encumbrances. This structure is intended to mitigate counterparty exposure associated with omnibus custody while maintaining operational controls and traceability over our reserve assets.
We conduct diligence and ongoing oversight of our wallet architecture and related service providers, focusing on segregation, key-management controls, access permissions, and incident-response procedures. Our controls emphasize least-privilege access, multi-factor authentication, operational separation of duties, and audit trails for key interactions and movement authorizations. We supplement these controls with periodic independent attestations to verify the existence, segregation, and control of our Bitcoin holdings as of specified dates.
For any client assets that may be onboarded to our platform in the future, we expect to utilize regulated third-party custodians and institutional-grade wallet solutions, implemented within a segregation and control framework designed to meet institutional compliance requirements (including AML/KYC, monitoring, and reporting). We anticipate diversifying custody solutions as appropriate to support risk management and operational resilience, and we will evaluate additional custodial partners over time.
Where we engage third-party providers, we expect to conduct initial and periodic reviews of their security, operations, and controls, including — but not limited to — key-management practices, cold-storage policies, cybersecurity programs, business continuity and disaster recovery readiness, and the availability of third-party assurance reporting. We also seek contractual provisions addressing segregation of assets and clarifying that digital assets held for our benefit are not part of a custodian’s bankruptcy estate under applicable law.
We continuously monitor the safekeeping of our Bitcoin through internal controls, reconciliation, and external confirmations, and we will conduct supplemental diligence when warranted by market conditions or other circumstances.
48
Policy Framework and Risk Management
We are building our platform around policy-based governance intended to satisfy institutional requirements and auditor expectations. Core elements include board-approved asset allocation and transaction policies, segregation of duties, multi-step approval workflows, transaction screening, real-time solvency and exposure limits, and emergency protocol “kill-switches.” For yield programs, we intend to maintain leverage caps, counterparty concentration limits, daily reconciliations, and tail-risk hedging parameters. For validator operations, we emphasize uptime targets, redundancy, slashing prevention, and pre-defined MEV/PBS participation rules. We expect to produce periodic “audit packs,” including reconciliations and control attestations, for institutional users.
Potential Advantages and Disadvantages of Holding Bitcoin
We believe that bitcoin is an attractive asset because it can serve as a store of value, supported by a robust and public open-source architecture, that is untethered to sovereign monetary policy. We also believe that, due to its limited supply, bitcoin offers the potential to serve as a hedge against inflation in the long-term and, if its adoption increases, the opportunity for appreciation in value.
Bitcoin exists entirely in electronic form, as virtually irreversible public transaction ledger entries on the blockchain, and transactions in bitcoin are recorded and authenticated not by a central repository, but by a decentralized peer-to-peer network. This decentralization mitigates the risks of certain threats common to centralized computer networks, such as denial-of-service attacks, and reduces the dependency of the bitcoin network on any single system. The decentralization of user nodes and miners also mitigates the risk of a 51% attack, which would be very costly and difficult to execute with respect to bitcoin because the Bitcoin network is open source and widely distributed, and transactions on the blockchain require significant computing power to be validated. However, while the Bitcoin network as a whole is decentralized, the private keys used to access bitcoin balances are not widely distributed and are susceptible to phishing and other attacks designed to obtain sensitive information or gain access to password-protected systems. Loss of such private keys can result in an inability to access, and effective loss of, the corresponding bitcoin. Consequently, bitcoin holdings are susceptible to all of the risks inherent in holding any electronic data, such as power failure, data corruption, security breach, communication failure and user error, among others. These risks, in turn, make bitcoin substantially more susceptible to theft, destruction, or loss of value from hackers, corruption, viruses and other technology-specific factors as compared to conventional fiat currency or other conventional financial assets.
In addition, the Bitcoin network relies on open-source developers to maintain and improve the Bitcoin protocol. Accordingly, bitcoin may be subject to protocol design changes, governance disputes such as “forked” protocols, competing protocols, and other open source-specific risks that do not affect conventional proprietary software.
Government Regulation
The laws and regulations applicable to bitcoin and digital assets are evolving and subject to interpretation and change.
Governments around the world have reacted differently to digital assets; certain governments have deemed them illegal, and others have allowed their use and trade without restriction, while in some jurisdictions, such as the U.S., digital assets are subject to overlapping, uncertain and evolving regulatory requirements.
As digital assets have grown in both popularity and market size, the U.S. Executive Branch, Congress and a number of U.S. federal and state agencies, including the Financial Crimes Enforcement Network (“FinCEN”), the Commodity Futures Trading Commission (“CFTC”), the SEC, the Financial Industry Regulatory Authority (“FINRA”), the Consumer Financial Protection Bureau (“CFPB”), the Department of Justice, the Department of Homeland Security, the Federal Bureau of Investigation, the Internal Revenue Service (“IRS”) and state financial regulators, have been examining the operations of digital asset networks, digital asset users and digital asset exchanges, with particular focus on the extent to which digital assets can be used to violate state or federal laws, including to facilitate the laundering of proceeds of illegal activities or the funding of criminal or terrorist enterprises, and the safety and soundness and consumer-protective safeguards of exchanges or other service-providers that hold, transfer, trade or exchange digital assets for users. Many of these state and federal agencies have issued consumer advisories regarding the risks posed by digital assets to investors. In addition, federal and state agencies, and other countries have issued rules or guidance regarding the treatment of digital asset transactions and requirements for businesses engaged in activities related to digital assets.
49
Depending on the regulatory characterization of bitcoin, the markets for bitcoin in general, and our activities in particular, our business and our bitcoin strategy may be subject to regulation by one or more regulators in the United States and globally. Ongoing and future regulatory actions may alter, to a materially adverse extent, the nature of digital assets markets, the participation of industry participants, including service providers and financial institutions in these markets, and our ability to pursue our bitcoin strategy. Additionally, U.S. state and federal and foreign regulators and legislatures have taken action against industry participants, including digital assets businesses, and enacted restrictive regimes in response to adverse publicity arising from hacks, consumer harm, or criminal activity stemming from digital assets activity. U.S. federal and state energy regulatory authorities are also monitoring the total electricity consumption of cryptocurrency mining, and the potential impacts of cryptocurrency mining to the supply and dispatch functionality of the wholesale grid and retail distribution systems. Many state legislative bodies have passed, or are actively considering, legislation to address the impact of cryptocurrency mining in their respective states.
The CFTC takes the position that some digital assets, including bitcoin, fall within the definition of a “commodity” under the Commodities Exchange Act of 1936, as amended (the “CEA”). Under the CEA, the CFTC has broad enforcement authority to police market manipulation and fraud in spot digital assets markets in which we may transact. Beyond instances of fraud or manipulation, the CFTC generally does not oversee cash or spot market exchanges or transactions involving digital asset commodities that do not utilize margin, leverage, or financing. In addition, CFTC regulations and CFTC oversight and enforcement authority apply with respect to futures, swaps, other derivative products and certain retail leveraged commodity transactions involving digital asset commodities, including the markets on which these products trade.
The SEC and its staff have taken the position that certain other digital assets fall within the definition of a “security” under the U.S. federal securities laws. Public statements made by senior officials and senior members of the staff at the SEC indicate that the SEC does not consider bitcoin to be a security under the federal securities laws. However, such statements are not official policy statements by the SEC and reflect only the speakers’ views, which are not binding on the SEC or any other agency or court and cannot be generalized to any other digital assets. In addition, since transactions in bitcoin provide a degree of anonymity, they are susceptible to misuse for criminal activities, such as money laundering. This misuse, or the perception of such misuse, could lead to greater regulatory oversight of bitcoin and Bitcoin platforms, and there is the possibility that law enforcement agencies could close or blacklist bitcoin platforms or other bitcoin-related infrastructure with little or no notice and prevent users from accessing or retrieving bitcoin held via such platforms or infrastructure. For example, the U.S. Treasury Department’s Office of Foreign Assets Control has issued updated advisories regarding the use of virtual currencies, added a number of digital asset exchanges and service providers to the Specially Designated Nationals and Blocked Persons list and engaged in several enforcement actions, including a series of enforcement actions that have either shut down or significantly curtailed the operations of several smaller digital asset exchanges associated with Russian and/or North Korean nationals. Additionally, in January 2025, the Consumer Financial Protection Bureau announced that it is seeking public input on privacy protections and surveillance in digital payments, particularly those offered through large technology platforms.
As noted above, activities involving bitcoin and other digital assets may fall within the jurisdiction of more than one financial regulator and various courts and such laws and regulations are rapidly evolving and increasing in scope. On January 23, 2025, President Trump issued an executive order titled, Strengthening American Leadership in Digital Financial Technology. While the executive order did not mandate the adoption of any specific regulations, the executive order identifies certain key objectives to guide agencies involved in crypto regulation, including (i) protecting the sovereignty of the United States dollar by promoting the development of United States dollar-backed stablecoins, (ii) providing regulatory clarity and certainty built on technology-neutral regulations for individuals and firms involved in digital assets, including through well-defined jurisdictional regulatory boundaries, and (iii) taking measures to protect Americans from the risks of Central Bank Digital Currencies. To achieve these objectives, the executive order established a working group on digital asset markets within the National Economic Council, comprised of representatives from key federal agencies, with a tight timeline for examining existing regulations and proposing a new regulatory framework. There have also been several bills introduced in Congress that propose to establish additional regulation and oversight of the digital asset markets.
Aspects of our business involve collecting, processing, disclosing, storing, and transmitting personal data, which are subject to certain privacy policies, contractual obligations, and U.S. and foreign laws, regulations, and directives relating to privacy and data protection.
50
There are a broad variety of other data protection laws in the United States that are or may be applicable to our activities, and a wide range of enforcement agencies at both the state and federal levels that can review companies for privacy and data security concerns based on general consumer protection laws. The Federal Trade Commission and state Attorneys General all are aggressive in reviewing privacy and data security protections for consumers. New laws also are being considered at both the state and federal levels. A broad range of legislative measures also have been introduced at the federal level. Accordingly, failure to comply with federal and state laws (both those currently in effect and future legislation) regarding privacy and security of personal information could expose us to fines and penalties under such laws. In the event of a security breach, we also may have obligations to notify our customers or other parties or individuals about this breach, and this can lead to significant costs and the risk of potential enforcement and/or litigation. There is also a threat of consumer class actions related to these laws and the overall protection of personal data. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our reputation and our business.
There are similar laws in other countries, including the General Data Protection Regulation (“GDPR”) in the European Union which imposes requirements regarding the handling and security of personal data, requires disclosure of data breaches to individuals, customers, and data protection authorities in certain circumstances, requires companies to honor data subjects’ requests relating to their personal data, permits regulators to impose fines of up to €20,000,000 or 4% of global annual revenue, whichever is higher, and establishes a private right of action.
In addition to these specific laws, we also are subject to other privacy, security, and data protection laws around the world. In addition to the laws in place already, other countries are also considering new or expanded laws governing privacy and data security that may impact our business practices. These laws may impact our ongoing business activities and our relationships with our business partners, customers and service providers.
Furthermore, the U.S. Congress is considering comprehensive privacy legislation. At this time, it is unclear whether Congress will pass such a law and if so, when and what it will require and prohibit. Moreover, it is not clear whether any such legislation would give the Federal Trade Commission (“FTC”) any new authority to impose civil penalties for violations of the Federal Trade Commission Act in the first instance, whether Congress will grant the FTC rulemaking authority over privacy and information security, or whether Congress will vest some or all privacy and data security regulatory authority and enforcement power in a new agency, akin to EU data protection authorities.
Sales and Marketing
Our sales and marketing strategy is designed to expand awareness of our institutional DAT platform, build credibility with corporate treasury teams and institutional allocators, and drive adoption of our offerings across target enterprise markets. Key elements include strategic partnerships, targeted distribution through enterprise sales and channels, and education-led marketing that emphasizes governance, compliance, and risk management.
Strategic Partnerships
We intend to rely on strategic relationships with regulated custodians, institutional-grade wallet providers, financial institutions, accounting and audit advisors, and other digital-asset service providers to support our treasury operations and client enablement. Because we are not directly licensed to provide custody, execution, or intermediation services, we expect these partners to supply the necessary regulatory infrastructure and, in certain cases, act as distribution channels. These relationships are expected to support secure custody, segregation and control frameworks, execution services for Bitcoin acquisitions and dispositions, validator operations, and programmatic risk management. We also expect to collaborate with placement agents and traditional financial institutions to broaden institutional reach and facilitate client onboarding. We will continue to evaluate additional partnerships with enterprise software integrators, cloud and security vendors, and protocol-level partners to enhance scale, resilience, and interoperability.
Distribution Methods
We plan to distribute our DAT solutions directly to enterprise clients through internal business development and relationship management teams focused on corporate treasuries, institutions, and financial intermediaries. In addition, we expect to leverage partnerships with custodians, wallet providers, and financial institutions to support client acquisition, onboarding, and ongoing operations. Over time, we anticipate supplementing these channels
51
with participation in industry conferences and education-led initiatives, including research and thought-leadership publications tailored for CFOs, treasury leaders, boards, and risk committees. We do not expect to rely on mass-market retail marketing programs.
Marketing
We intend to emphasize education, transparency, and strategic engagement aligned with institutional standards. We expect to target the following principal audiences:
• Corporate treasury managers and CFO organizations seeking to integrate Bitcoin into reserve strategies with institutional governance, controls, and reporting.
• Institutional investors and asset managers, including hedge funds and family offices, seeking institutional-grade custody, risk-managed yield programs, and validator-enabled capabilities.
• Financial institutions and intermediaries evaluating Bitcoin treasury offerings for their clients and seeking compliant, scalable infrastructure.
• High-net-worth individuals with significant Bitcoin holdings who require institutional-grade custody, governance, and programmatic operations.
Our marketing strategy is expected to combine targeted enterprise outreach, channel partnerships, and education-driven content intended to promote responsible adoption of institutional Bitcoin treasury practices while reinforcing MindWave’s commitment to compliance, governance, and long-term value creation.
Intellectual Property
We rely on a combination of proprietary software, trade secrets, and contractual protections to develop and operate our DAT platform, including components related to wallet orchestration, segregation and controls, risk analytics, AI-supported yield programs, and validator operations. We also rely on confidentiality and invention-assignment agreements with employees, contractors, and partners. From time to time, we may pursue trademark protection for brand names and logos associated with our products and ecosystem (including, for example, MindWave and $NILA), and we will evaluate additional intellectual property protections as our platform develops and markets evolve.
Seasonality
Our business is not subject to material seasonal variations. However, our results of operations may be affected by fluctuations in the market price of bitcoin and by the timing of capital raising activities, which may not occur evenly throughout the year.
Employees
As of [January 11], 2026, we had a total of [09] employees, [0] of whom are based in the United States. [0] of our employees are represented by a labor union. We have not experienced any work stoppages and generally consider our relations with our employees to be good.
Legal Proceedings
From time to time, we may be involved in legal proceedings, claims, or regulatory matters arising in the ordinary course of business. As of the date of this registration statement, we [are/not] a party to any material pending legal proceedings, nor are we aware of any such proceedings contemplated by governmental authorities.
Facilities
[—]
Available Information
Our principal executive offices are located at 60 Paya Lebar Road #04-23, Singapore 409051. Our website is located at www.mindwavedao.com.
52
MINDWAVE INNOVATIONS INC’S MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATION
References in this section to “we,” “us” or the “Company” refer to MindWave Innovations Inc. References to our “management” or our “management team” refer to our officers and directors. Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States (“GAAP”). These accounting principles require us to make certain estimates, judgments and assumptions. We believe that the estimates, judgments and assumptions upon which we rely are reasonable based upon information available to us at the time that these estimates, judgments and assumptions are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities as of the date of the consolidated financial statements as well as the reported amounts of revenues and expenses during the periods presented. Our consolidated financial statements would be affected to the extent there are material differences between these estimates and actual results. In many cases, the accounting treatment of a particular transaction is specifically dictated by GAAP and does not require management’s judgment in its application. There are also areas in which management’s judgment in selecting any available alternative would not produce a materially different result. The following discussion should be read in conjunction with our consolidated financial statements and notes thereto.
Overview
MindWave Innovations Inc. is an early-stage digital asset and technology company focused on providing institutional digital asset treasury (“DAT”) solutions. Our strategy centers on compliant bitcoin treasury infrastructure, AI-driven yield capabilities, ClimateTech impact systems, and AdTech engagement platforms, supported by a blockchain-enabled ecosystem utilizing our native token, $NILA.
Mindwave Innovations Inc. (the “Company”) was incorporated under the laws of the State of Delaware on November 10, 2025 to become the ultimate holding company of TechyTrade FZ-LLC, a company incorporated in United Arab Emirates, and TechyTrade Innovations Pte. Ltd, a company incorporated in Singapore. TechyTrade Innovations Pte. Ltd. (“TechyTrade Singapore”) was incorporated in Singapore on September 8, 2025. TechyTrade FZ-LLC was originally a company incorporated in United Arab Emirates on December 27, 2020 and is the entity that maintains historical operations of the Company.
The Company is a leading provider of institutional Digital Asset Treasury (DAT) solutions, specializing in compliant Bitcoin treasury infrastructure, AI-driven yield capabilities, ClimateTech impact systems, and AdTech engagement platforms. The company’s multi-vertical ecosystem is powered by its native token, $NILA, which enables governance, utility, and value flow across its blockchain-integrated operations.
MindWave is a global leader in AI-driven Bitcoin and yield-generation technologies, operating in one of the fastest-growing segments of the digital-asset market. Bitcoin remains the most established and highly valued cryptocurrency, and MindWave’s platform is designed to help institutions securely hold, manage, and generate yield from Bitcoin reserves.
MindWave’s three-pronged strategic framework includes:
1. Secure Digital Treasury Wallets for Corporations,
2. AI-Enhanced Bitcoin Yield Generation, and
3. A Validator-Powered Ecosystem supported by the $NILA Token. Together, these capabilities make
MindWave one of the first companies to pursue a publicly traded, institutional-focused Digital Asset Treasury (DAT) model.
Reorganization
Prior to the reorganization transaction described below, TechyTrade FZ-LLC maintained historical operarations of the Company since its formation in 2020. In September 2025, TechyTrade FZ-LLC ebcame a wholly owned subsidiary of TechyTrade Innovations Pte. Ltd.
53
On November 10, 2025, Mindwave Innovations Inc. entered into a Reorganization Agreement and Plan of Share Exchange to consolidate its corporate structure. Under this agreement, the Company issued 1,001 shares of its common stock in exchange for 100% of the membership interests in TechyTrade FZ-LLC, which was previously held by TechyTrade Singapore. As a result of this transaction, TechyTrade FZ-LLC became a wholly owned subsidiary of Mindwave Innovations Inc., establishing the Company as the ultimate holding entity for the group.
The Reorganization is being accounted for as a reorganization of entities under common control. The accompanying financial statements have been presented to retroactively present the effect of the Reorganization. See Note 3 for further detail.
Merger with Apimeds Pharmaceuticals US, Inc.
On December 1, 2025, MindWave Innovations Inc. (the “Company”) completed a strategic business combination pursuant to an Agreement and Plan of Merger (the “Merger Agreement”) among the Company, Apimeds Merger Sub, Inc., Apimeds Pharmaceuticals US, Inc. (“Apimeds”), and other parties thereto. Pursuant to the Merger Agreement and in accordance with the Delaware General Corporation Law, Apimeds Merger Sub, Inc. merged with and into Apimeds, with Apimeds surviving the merger as a wholly owned subsidiary of the Company. The merger became effective upon the filing of a certificate of merger with the Secretary of State of the State of Delaware, and the closing occurred simultaneously with the execution of the Merger Agreement.
At the effective time of the merger, all outstanding shares of the Company’s common stock were converted into the right to receive merger consideration consisting of shares of the Company’s preferred stock and a limited number of shares of the Company’s common stock, subject to applicable caps. On an as-converted and fully diluted basis, the former stockholders of the Company collectively own approximately 90.9% of the outstanding equity securities of the combined company immediately following the closing. As a result of the merger, Apimeds’ assets, liabilities, and operations are included in the Company’s consolidated financial statements, and the transaction resulted in a significant change in the Company’s capital structure and ownership.
The merger was undertaken to combine the Company’s digital asset treasury and technology platform with Apimeds’ clinical-stage biopharmaceutical operations. Management believes the transaction provides an expanded corporate platform; however, the Company’s future results will depend on the successful integration of the businesses, the execution of its revised business strategy, and the availability of additional capital. Additional information regarding the Merger Agreement and the related transactions is included in the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 10, 2025.
Convertible Note Financing
In connection with the closing of the merger, on December 1, 2025, the Company entered into a Securities Purchase Agreement with an institutional investor providing for the issuance of senior unsecured convertible notes in an aggregate principal amount of up to $120.9 million over a 24-month commitment period. At the initial closing, $10.9 million of principal amount was made available to the Company, with additional amounts subject to the effectiveness of a resale registration statement and the investor’s election to fund additional tranches.
The notes bear no interest absent an event of default and mature 12 months from issuance at 100% of face value. The notes are convertible into shares of the Company’s common stock at a variable conversion price based on a discount to recent trading prices, subject to monthly conversion limitations. The Company is required to obtain stockholder approval for the issuance of shares upon conversion of the notes and has entered into voting agreements with certain officers, directors, and stockholders in support of such approval. The financing includes customary covenants and registration rights, and failure to meet certain registration effectiveness deadlines may result in liquidated damages.
The Company expects to use the net proceeds from the note financing to fund post-merger operations, integration activities, working capital, and general corporate purposes. Additional information regarding the Securities Purchase Agreement and related amendments is disclosed in the Company’s Current Reports on Form 8-K filed on December 2, 2025 and December 10, 2025.
54
Bitcoin Treasury Strategy and Accounting Treatment
Digital assets are considered intangible assets under Accounting Standards Codification Subtopic 350-60, Intangibles — Goodwill and Other — Crypto Assets (“ASC 350-60”) and Accounting Standards Update 2023-08. Under ASC 350-60, digital assets that meet the definition of intangible assets, among other criteria, are initially recorded at cost and are then subsequently remeasured at fair value, as of the balance sheet date with changes from remeasurement recognized in net income. The fair value for digital assets that are unrestricted (“liquid”) and freely tradable are determined using Level 1 inputs as defined in ASC 820. Conversely, fair value for digital assets that are restricted (“locked”) due to vesting schedules or contractual restrictions are determined based on Level 1 inputs adjusted for the impact of such restrictions based on Level 2 inputs, in accordance with ASC 820. We account for changes from remeasurement within (gain) loss from changes in fair value of digital assets, as stated on our consolidated statements of operations.
Digital assets that we hold that do not meet the criteria under ASC 350-60 are accounted for as intangible assets with indefinite useful lives and are initially recorded at cost and are not amortized but instead are tested for impairment at least annually, or more frequently, if events or changes in circumstances indicate that it is more likely than not that the asset is impaired. As such, these digital assets are reflected in our balance sheets, at cost net of any impairments within Digital assets, at carrying value. We evaluate impairments on these digital assets on at least an annual basis, or more frequently if events or changes in circumstances occur and use Level 1 inputs, unadjusted quoted prices in active markets, for the fair value to determine if an impairment has occurred. If unadjusted quoted prices in active markets for our digital assets are lower than the carrying value recorded, then we will record an impairment charge equal to the difference between the carrying value and the fair value. Impairment charges are recognized within (Gain) loss on changes in fair value of digital assets, as stated on our consolidated statements of operations.
When we receive digital assets earned on third party staking platforms, we will initially record them at fair value on the date the digital assets are received. Upon the disposal of digital assets, any gains or losses are recognized in realized gain on sale of digital assets, as stated on our consolidated statements of operations, and are calculated as the difference between the selling price and our carrying value, using the specific identification method. See Note 6 — Digital Assets for further discussion.
Market-making Activities
The Company accounts for its market-making activities in accordance with ASC 350-60, Crypto Assets. Digital assets are measured at fair value at each reporting date, with changes in fair value recognized in operating income. The Company classifies these assets as Level 1 within the fair value hierarchy per ASC 820, utilizing unadjusted quoted prices from the Lbank exchange.
In accordance with ASC 350-60-45-1, realized gains and losses from trading are presented as trading (gains) losses, net within operating income.
Components of Operating Results
Revenue
We earn revenue from consulting services to customers.
Cost of Revenues
Cost of revenue includes direct costs and costs related to marketing and technical consultancy.
Operating Expenses
General and Administrative
General and administrative expenses primarily consist of administrative costs and marketing and promotional expenses related to digital assets. Pursuant to a service agreement (see Note 10), the Company receives legal, administrative support, compliance (KYC/AML), reporting and security services for the underlying NILA tokens. These costs are expensed as incurred.
55
Distribution and Commission
Distribution and commission expenses primarily consist of deduction and payment of 44% of gross token sale proceeds as commission to the Distributor. Pursuant to a service agreement (see Note 10), the Distributor is committed to exclusive sales and collection of NILA tokens. These costs are expensed as incurred.
Realized (gain) loss on sale of digital assets
Upon the disposal of digital assets, any gains or losses are recognized in realized gain on sale of digital assets, as stated on our consolidated statements of operations, and are calculated as the difference between the selling price and our carrying value, using the specific identification method. See Note 6 — Digital Assets for further discussion.
Unrealized (gain) loss from changes in fair value of digital assets
We account for changes from remeasurement within (gain) loss from changes in fair value of digital assets, as stated on our consolidated statements of operations.
Results of Operations
Comparison of Six Months Ended September 30, 2025 and 2024
The following table sets forth key components of our results of operations for the six months ended September 30, 2025 and 2024, both in dollars and as a percentage of change.
|
Six Months Ended |
Change |
Percent |
|||||||||||||
|
2025 |
2024 |
||||||||||||||
|
Revenues |
$ |
— |
|
$ |
— |
|
$ |
— |
|
0 |
% |
||||
|
|
|
|
|
|
|
|
|||||||||
|
Operating expense (income): |
|
|
|
|
|
|
|
||||||||
|
General and administrative |
|
3,371,133 |
|
|
2,273,557 |
|
|
1,097,576 |
|
48 |
% |
||||
|
Distribution and commission expenses |
|
4,634,172 |
|
|
3,261,257 |
|
|
1,372,915 |
|
42 |
% |
||||
|
Trading gains, net |
|
(3,300 |
) |
|
— |
|
|
(3,300 |
) |
100 |
% |
||||
|
Realized gain on sale of digital assets |
|
(10,479,790 |
) |
|
(7,333,122 |
) |
|
(3,146,668 |
) |
43 |
% |
||||
|
Unrealized gain from changes in fair value of digital assets |
|
9,066,549 |
|
|
(56,026,550 |
) |
|
65,093,099 |
|
-116 |
% |
||||
|
Total operating expense (income) |
|
6,588,764 |
|
|
(57,824,858 |
) |
|
64,413,622 |
|
-111 |
% |
||||
|
(Loss) income from operations |
|
(6,588,764 |
) |
|
57,824,858 |
|
|
(64,413,622 |
) |
-111 |
% |
||||
|
Net (loss) income |
$ |
(6,588,764 |
) |
$ |
57,824,858 |
|
$ |
(64,413,622 |
) |
-111 |
% |
||||
General and Administrative
General and administrative expenses increased by $1,097,576 for the six months ended September 30, 2025, compared to the same period in the prior year. This variance was primarily driven by an escalation in transactional and related costs incurred in connection with the Company’s strategic sale of digital assets. These expenses are directly correlated with the volume and frequency of digital asset liquidations executed to meet the Company’s operational and liquidity objectives during the reporting period.
Distribution and Commission Expenses
Distribution and commission expenses experienced a significant upward adjustment, increasing by $1,372,915 for the six months ended September 30, 2025, as compared to the corresponding period in the prior year. This variance was primarily driven by an escalation in transactional and related costs incurred in connection with the Company’s strategic sale of digital assets. These expenses are directly correlated with the volume and frequency of digital asset liquidations executed to meet the Company’s operational and liquidity objectives during the reporting period.
56
Trading Gains, Net
Net trading gains from trading in market making income during the six months ended September 30, 2025 was $3,300.
Net (Loss) Income
Net loss was $6,588,764 for the six months ended September 30, 2025 as compared to net income of $57,824,858, a decrease of $64,413,622. The decrease was primarily due to lower unrealized gain from changes in fair value of digital assets in 2025 as compared to the prior period.
Comparison of Years Ended March 31, 2025 and 2024
The following table sets forth key components of our results of operations for the years ended March 31, 2025 and 2024, both in dollars and as a percentage of change.
|
Year Ended |
Change |
Percent |
||||||||||||
|
2025 |
2024 |
|||||||||||||
|
Revenue |
$ |
— |
|
$ |
621,169 |
$ |
(621,169 |
) |
-100 |
% |
||||
|
Cost of revenue |
|
— |
|
|
83,895 |
|
(83,895 |
) |
-100 |
% |
||||
|
Gross profit |
$ |
— |
|
$ |
537,274 |
$ |
(537,274 |
) |
-100 |
% |
||||
|
Operating (income) expense: |
|
|
|
|
|
|
||||||||
|
General and administrative |
|
2,674,157 |
|
|
111,348 |
|
2,562,809 |
|
2302 |
% |
||||
|
Distribution and commission expenses |
|
3,718,158 |
|
|
— |
|
3,718,158 |
|
100 |
% |
||||
|
Realized gain on sale of digital assets |
|
(8,360,488 |
) |
|
— |
|
(8,360,488 |
) |
100 |
% |
||||
|
Unrealized gain from changes in fair value of digital assets |
|
(53,930,184 |
) |
|
— |
|
(53,930,184 |
) |
100 |
% |
||||
|
Total operating (income) expense |
|
(55,898,357 |
) |
|
111,348 |
|
(56,009,705 |
) |
-50302 |
% |
||||
|
Income from operations |
|
55,898,357 |
|
|
425,927 |
|
55,472,430 |
|
13024 |
% |
||||
|
Other income (expense): |
|
— |
|
|
32,007 |
|
(32,007 |
) |
-100 |
% |
||||
|
Net income |
$ |
55,898,357 |
|
$ |
457,933 |
$ |
55,440,424 |
|
12107 |
% |
||||
General and Administrative
General and administrative expenses increased by $2,562,809 for the year ended March 31, 2025, compared to the same period in the prior year. This variance was primarily driven by an escalation in transactional and related costs incurred in connection with the Company’s strategic sale of digital assets. These expenses are directly correlated with the volume and frequency of digital asset liquidations executed to meet the Company’s operational and liquidity objectives during the reporting period.
Distribution and Commission Expenses
Distribution and commission expenses experienced a significant upward adjustment, increasing by $3,718,158 for the year ended March 31, 2025, as compared to the corresponding period in the prior year. This variance was primarily driven by an escalation in transactional and related costs incurred in connection with the Company’s strategic sale of digital assets. These expenses are directly correlated with the volume and frequency of digital asset liquidations executed to meet the Company’s operational and liquidity objectives during the reporting period.
Net Income
Net income was $55,898,357 for the year ended March 31, 2025 as compared to net income of $457,933, an increase of $55,440,424. The increase was primarily due to realized gain on sale of digital assets and unrealized gain from changes in fair value of digital assets in 2025 as compared to the prior year.
57
Liquidity and Capital Resources
Our ability to meet future liquidity needs depends on several factors, including the market value of our bitcoin holdings, our ability to access equity and debt financing on acceptable terms, and our operating cash requirements. Because bitcoin does not generate cash flows, we may be required to sell bitcoin, pledge bitcoin as collateral, or raise additional capital to fund operations, service debt obligations, or pursue strategic initiatives. Any such actions could expose us to market volatility, dilution, or unfavourable financing terms.
Management believes that existing liquidity, together with anticipated access to capital markets, will be sufficient to fund operations for at least the next twelve months. However, adverse market conditions, declines in bitcoin prices, regulatory developments, or unexpected expenses could materially impact our liquidity position.
Cash Flows
Six months ended September 30, 2025 and 2024
The following is a summary of the Company’s cash flows provided (used in) operating and investing activities:
|
Six Months Ended |
||||||||
|
2025 |
2024 |
|||||||
|
Net cash used in operating activities |
$ |
(8,004,479 |
) |
$ |
(5,534,672 |
) |
||
|
Net cash provided by investing activities |
$ |
8,004,479 |
|
$ |
5,534,671 |
|
||
|
Net change in cash |
$ |
— |
|
$ |
(1 |
) |
||
Net Cash Used in Operating Activities
Cash used in operating activities was $8,004,479 for the six months ended September 30, 2025 as compared to $5,534,672 for the six months ended September 30, 2024. Cash used during the six months ended September 30, 2025 was primarily due to our net loss of $6,588,764, non-cash charges of $1,412,415 and cash used in operating assets and liabilities of $3,300. Cash used during the six months ended September 30, 2024 was primarily due to our net income of $57,824,858, offset by non-cash charges of $63,359,530.
Net Cash Provided by Investing Activities
Cash provided by investing activities was $8,004,479 and $5,534,671 for the six months ended September 30, 2025 and 2024, respectively. Investing activities in 2025 were primarily due to sale of digital assets of $10,532,210, partially offset by purchase of digital assets of $2,527,730. Investing activities in 2024 were primarily due to sale of digital assets of $7,394,745, offset by purchase of digital assets of $1,860,073.
The following is a summary of the Company’s cash flows provided (used in) operating, investing and financing activities:
|
Year Ended |
||||||||
|
2025 |
2024 |
|||||||
|
Net cash (used in) provided by operating activities |
$ |
(6,392,032 |
) |
$ |
422,779 |
|
||
|
Net cash provided by investing activities |
$ |
6,392,031 |
|
$ |
— |
|
||
|
Net cash used in financing activities |
$ |
— |
|
$ |
(423,254 |
) |
||
|
Net change in cash |
$ |
(1 |
) |
$ |
(475 |
) |
||
Net Cash (Used in) Provided by Operating Activities
Cash (used in) provided by operating activities was $(6,392,032) for the year ended March 31, 2025 as compared to $422,779 for the year ended March 31, 2024. Cash used during the year ended March 31, 2025 was primarily due to our net income of $55,898,357, offset by non-cash charges of $62,290,389. Cash used during the year ended March 31, 2024 was primarily due to our net income of $457,933, partially offset by non-cash charges of $31,724 and cash used in operating assets and liabilities of $3,431.
58
Net Cash Provided by Investing Activities
Cash provided by investing activities was $6,392,031 and $0 for the years ended March 31, 2025 and 2024, respectively. Investing activities in 2025 were primarily due to sale of digital assets of $8,435,804, offset by purchase of digital assets of $2,043,773.
Net Cash Used in Financing Activities
Cash used in financing activities was $423,254 for the year ended March 31, 2024. Which was primarily due to repayment of a related party loan.
Critical Accounting Policies and Significant Judgments and Estimates
This discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with generally accepted accounting standards in the United States (“GAAP”). The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. While our significant accounting policies are described in more detail in the notes to our financial statements included elsewhere in this prospectus, we believe that the following accounting policies are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
We believe our most critical accounting policies and estimates relate to the following:
Revenue Recognition
The Company recognizes revenue from services in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) Topic 606, Revenue from Contracts with Customers (“ASC 606”). Under ASC 606, the Company recognizes revenue when or as the Company’s performance obligations are satisfied by transferring control of the promised services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those services. To determine revenue recognition for arrangements that an entity determines are within the scope of ASC 606, the Company performs the following five steps as prescribed by ASC 606:
(i) identify the contract(s) with a customer;
(ii) identify the performance obligations in the contract;
(iii) determine the transaction price;
(iv) allocate the transaction price to the performance obligations in the contract; and
(v) recognize revenue when (or as) the entity satisfies performance obligations.
The Company only applies the five-step model to contracts when it is probable that it will collect substantially all the consideration it is entitled to in exchange for the goods or services it transfers to the customer. Once the contract is determined to be within the scope of ASC 606, the Company assesses the goods or services promised within each contract, the performance obligations in each contract and whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied. The Company has applied ASC 606 on a portfolio basis. The Company has elected the practical expedients, allowing the recognition of incremental costs of obtaining a contract as an expense when incurred, and not adjust the transaction price for the effects of a significant financing component if, at contract inception, the period between customer payment and the transfer of goods or services is expected to be one year or less.
59
Recently Issued and Adopted Accounting Pronouncements
A description of recently issued and adopted accounting pronouncements that may potentially impact our financial position and results of operations is disclosed in Note 3 to our consolidated financial statements.
Off-Balance Sheet Arrangements
During the periods presented, we did not have, nor do we currently have, any off-balance sheet arrangements as defined under SEC rules.
Quantitative and Qualitative Disclosures about Market Risk
Market Risk
The Company is exposed to market risk related to the digital asset holdings, which are impacted by the market value of the respective underlying digital asset. The Company performed a sensitivity analysis assuming a hypothetical 10% change in the fair value of these digital assets to demonstrate the potential impact on the financial results. A hypothetical 10% increase or decrease in market prices would have positively or negatively impacted Income (loss) before income taxes by approximately $13.2 million for the six months ended September 30, 2025.
Interest Rate Risk
Our cash consists of cash in readily available checking accounts. We may also invest in short-term money market fund investments. Such interest-earning instruments carry a degree of interest rate risk, however, historical fluctuations in interest income have not been significant.
Inflation Risk
Inflation generally affects us by increasing our cost. We do not believe inflation has had a material effect on our results of operations during the periods presented.
60
Risks Related to Our Financial Position and Capital Needs
We are in the early stages of clinical development for our product candidate Apitox, which may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
To date, we have devoted substantially all of our resources to performing research and development, undertaking preclinical and clinical studies and enabling manufacturing activities in support of our product development efforts, hiring personnel, acquiring and developing our technology, performing business planning, establishing our intellectual property portfolio and raising capital to support and expand such activities. As an organization, we have not yet demonstrated an ability to conduct sales and marketing activities necessary for successful commercialization or arrange for a third party to conduct these activities on our behalf. Consequently, any predictions about our future success or viability may not be as accurate as they could be if we had a longer operating history.
Our current portfolio includes one product candidate, and we do not expect to generate revenue from our product candidate in the near future. We may encounter unforeseen expenses, difficulties, complications, delays and other known or unknown factors in achieving our business objectives, including with respect to our clinical candidate. We are transitioning from an early stage research and development company to late stage development company, with a focus on delivering Phase III clinical data establishing Apitox as a viable commercial candidate. We may not be successful in this transition.
We have incurred significant net losses since inception and anticipate that we will continue to incur substantial net losses for the foreseeable future and may never achieve profitability.
We are a clinical stage biopharmaceutical company that was formed on May 11, 2020. Investment in clinical stage companies is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential product candidates will not gain regulatory approval or become commercially viable. We have not generated any revenue from product sales. As a result, we are not profitable and have incurred losses in each year since inception. Our net losses were $1,389,990 and $777,694 for the years ended December 31, 2024 and 2023, respectively. As of September 30, 2025, we had an accumulated deficit of $9,237,769.
We expect to continue to spend significant resources to fund research and development of, and seek regulatory approvals for, our product candidate. We expect to incur substantial and increasing operating losses over the next several years. As a result, our accumulated deficit will also increase significantly. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenue. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital. We may never be profitable and, if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
We will require substantial additional funding to finance our operations. If we are unable to raise additional capital when needed, we could be forced to delay, reduce or terminate certain of our development programs or other operations.
As of September 30, 2025, we had cash of $6,986,617. We believe that the funds from the Note Financing, together with our existing cash as of the date of this Information Statement, will fund our current operating plans through at least the next 12 months from the date of this Information Statement. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned. We expect to finance our cash needs through public or private equity or debt financings, third-party (including government) funding and marketing and distribution arrangements, as well as other collaborations, strategic alliances and licensing arrangements or any combination of these approaches. Our ability to raise additional capital may be
61
adversely impacted by potential worsening global economic conditions and the recent disruptions to and volatility in the credit and financial markets in the United States and worldwide, including the trading price of common stock. Our future capital requirements will depend on many factors, including:
• the timing, scope, progress, results and costs of research and development, testing, screening, manufacturing, preclinical development and clinical trials;
• the outcome, timing and cost of seeking and obtaining regulatory approvals from the U.S. Food and Drug Administration, or the FDA;
• our ability to maintain existing, and establish new, strategic collaborations, licensing or other arrangements and the financial terms of any such agreements, including the timing and amount of any future milestone, royalty or other payments due under any such agreement;
• any product liability or other lawsuits related to our products;
• the expenses needed to attract, hire and retain skilled personnel;
• the identification and pursuit of additional clinical or regulatory opportunities;
• the costs to establish, maintain, expand, enforce and defend the scope of our intellectual property portfolio, including the amount and timing of any payments we may be required to make, or that we may receive, in connection with licensing, preparing, filing, prosecuting, defending and enforcing of any patents or other intellectual property rights; and
• the costs of operating as a public company.
Our ability to raise additional funds will depend on financial, economic and other factors, many of which are beyond our control. We cannot be certain that additional funding will be available on acceptable terms, or at all. We have no committed source of additional capital and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development of our product candidate or other research and development initiatives. Our license agreements may also be terminated if we are unable to meet the payment obligations or milestones under the agreements. We could be required to seek collaborators for our product candidate at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available or relinquish or license on unfavorable terms our rights to our products in markets where we otherwise would seek to pursue development ourselves.
Raising additional capital may cause dilution to our stockholders, including the Investor, restrict our operations or require us to relinquish rights to one or more of our technologies.
We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, intellectual property, future revenue streams, research programs or current or future product candidates or grant licenses on terms unfavorable to us and our stockholders.
The report of our independent registered public accounting firm included a “going concern” explanatory paragraph.
The report of our independent registered public accounting firm on our financial statements as of and for the years ended December 31, 2024 and 2023 included an explanatory paragraph indicating that there was substantial doubt about our ability to continue as a going concern. If we are unable to raise additional capital as and when needed, our business, financial condition and results of operations will be materially and adversely affected, and we may be forced to delay our development efforts, limit our activities and reduce research and development costs.
62
If we are unable to continue as a going concern, we may have to liquidate our assets, and the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements. The inclusion of a going concern explanatory paragraph by our independent registered public accounting firm, our lack of cash resources and our potential inability to continue as a going concern may materially adversely affect our share price and our ability to raise new capital, enter into licensing and collaboration arrangements or other contractual relationships with third parties and otherwise execute our development strategy.
We have identified material weaknesses in our internal control over financial reporting, and the failure to remediate these material weaknesses may adversely affect our business, investor confidence in our company, our financial results and the market value of our common stock.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. As disclosed in our Quarterly Report on Form 10-Q for the period ended September 30, 2025, filed with the SEC on November 12, 2025 (the “Quarterly Report”), our management conducted an assessment of the effectiveness of our internal control over financial reporting as of September 30, 2025, based on the framework established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO Framework). Management identified a material weakness in our internal control over financial reporting. Specifically, the Company does not currently have sufficiently documented procedures or control activities in place to support a reliable financial reporting process. This includes an absence of controls over the review and approval of journal entries, segregation of duties, reconciliations, and other fundamental accounting processes.
The material weakness did not result in any material misstatements to the Company’s financial statements, and management has concluded that the Company’s financial statements and other financial information included in its Quarterly Report fairly and accurately present the Company’s financial condition, results of operations, and cash flows for the periods in accordance with GAAP.
We have begun exploring remedial efforts to address the underlying causes of the material weaknesses. There can be no assurance that any remedial efforts we take, if any, will be sufficient to remediate the control deficiencies that led to our material weaknesses in our internal controls over financial reporting or prevent future material weaknesses or control deficiencies from occurring.
If the Company fails to remediate the material weaknesses or any future deficiencies, or fails to otherwise maintain the adequacy of its internal controls, that could result in a restatement of the Company’s financial statements for prior periods, a decline in the market value of the Company’s common stock, one or more investigations or enforcement actions by state or federal regulatory agencies, stockholder lawsuits, or other adverse actions requiring the Company to incur defense costs or pay fines, settlements, or judgments.
Risks Related to Our Business and Industry
Our business is substantially dependent on our relationships with our principal stockholder, Apimeds Korea. The loss of this relationship would have a material adverse effect on our business.
We are dependent on a license from our principal stockholder, Apimeds Korea, to continue our clinical trials and development of Apitox. We have entered into a license agreement with Apimeds Korea to obtain exclusive rights in the United States to the principal trade secrets, know-how and data relating to Apitox. If we default or fail to perform any of our obligations under this agreement, Apimeds Korea may terminate the agreement, subject to advance notice to cure such default. Any termination of this license agreement would have a materially adverse impact on our business and prospects.
There may be conflicts of interest amongst our directors and officers and Apimeds Korea.
Conflicts may arise between our directors and officers and Apimeds Korea, as some of our directors and officers hold positions with both companies. Mr. Jakap Koo, one of our directors, is the Chief Executive Officer of and President of Apimeds Korea and of Apimeds Korea’s parent company, Inscobee Inc. These conflicts could discourage the parties from working collaboratively and our commercial success will be dependent, in part, upon the performance of our management.
63
Although such officers and directors are aware of their duties and accountability to our Company and to applicable laws and policies relating to corporate opportunity and conflicts of interest, such conflicts of interest may include deciding how much time to devote to our affairs, as well as what business opportunities should be presented to us. Additionally, any dispute that may arise between us and Apimeds Korea may make it more difficult to favorably resolve such disputes.
The Company is reliant on its key supplier.
We contract directly with a United States company for the supply of dried bee venom and have exclusivity in the field of pharmaceutical use, which does not include using venom for immunology, cosmetic or any other non-pharmaceutical use, for a period of ten years from November 3, 2021. The exclusivity exception is for sales of bee venom to Apimeds Korea for use outside the United States. The agreement may be terminated upon mutual written consent of both parties. Termination of this agreement, variations in their terms or the failure of our key supplier to comply with its obligations under its agreement (including if our key supplier were to become insolvent) could have a material adverse effect on the Company’s consolidated financial results and on your investment.
As an organization, we have limited experience designing and implementing clinical trials, and we have never conducted pivotal clinical trials. Failure to adequately design a trial, or incorrect assumptions about the design of the trial, could adversely affect the ability to initiate the trial, enroll patients, complete the trial, or obtain regulatory approval on the basis of the trial results, as well as lead to increased or unexpected costs and in delayed timelines.
The design and implementation of clinical trials is a complex process. We have limited experience designing and implementing clinical trials, and we may not successfully or cost-effectively design and implement clinical trials that achieve our desired clinical endpoints efficiently, or at all. A clinical trial that is not well designed may delay or prevent initiation or completion of the trial, which can lead to increased difficulty in enrolling patients, may make it more difficult to obtain regulatory approval for the product candidate on the basis of the study results, or, even if a product candidate is approved, could make it more difficult to commercialize the product successfully or obtain reimbursement from third-party payors. Additionally, a trial that is not well-designed could be inefficient or more expensive than it otherwise would have been, or we may incorrectly estimate the costs to implement the clinical trial, which could lead to a shortfall in funding. If we select an incorrect dose or dose administration schedule, that could negatively impact the results of the trial, including if we select doses that are too low to be effective or administer doses too infrequently based on the half-life of the active ingredient. We also expect to continue to rely on third parties to conduct our pivotal clinical trials. See “— Risks Related to Reliance on Manufacturing and Third Parties.” If these third parties do not successfully carry out their contractual duties, comply with regulatory requirements or meet expected deadlines, we may not be able to obtain marketing approval for or commercialize Apitox or any future product candidates we develop, and our business could be materially harmed. Consequently, we may be unable to successfully and efficiently execute and complete clinical trials that are required for BLA submission and the FDA approval of Apitox or future product candidates. We may require more time and incur greater costs than our competitors and may not succeed in obtaining regulatory approvals of product candidates that we develop.
If we are unable to successfully develop, receive regulatory approval for, and commercialize our product candidate or future product candidates, our business will be harmed.
Our product candidate is still in preclinical and clinical development, and we are early in our development efforts. The FDA permitted our investigational new drug application for Apitox to proceed in 2014, and we began enrolling subjects. Our product candidate will require additional preclinical and/or clinical development, regulatory approval, obtaining manufacturing supply, capacity, and expertise, building a commercial organization or successfully outsourcing commercialization, substantial investment, and significant marketing efforts, before we generate any revenue from product sales. We do not have any products that are approved for commercial sale, and we may never be able to develop or commercialize marketable products.
Our ability to generate revenue from our product candidate, which we do not expect will occur for several years, if ever, will depend heavily on the successful development, regulatory approval, and eventual commercialization of our product candidate. The success of our product candidate or any other product candidates that we develop or otherwise may acquire will depend on several factors, including:
• timely and successful completion of preclinical studies and clinical trials;
• effective INDs submitted to the FDA that allow commencement of our planned clinical trials or future clinical trials for our product candidate;
64
• sufficiency of our financial and other resources to complete the necessary preclinical studies and clinical trials;
• successful development of, or making arrangements with third-party manufacturers for, our commercial manufacturing processes for any of our product candidates that receive regulatory approval;
• making arrangements for the manufacturing of our product candidate for our clinical trials, and manufacturing our product candidate at an acceptable cost and on a timely basis;
• receipt of timely marketing approvals from the FDA;
• launching commercial sales of products, if approved;
• acceptance of the benefits and use of our products, if approved, by patients, the medical community, and third-party payors, for their approved indications;
• the prevalence and severity of adverse events or other safety issues experienced with our product candidate;
• the availability, perceived advantages, cost, safety, and efficacy of alternative therapies for any product candidate, and any indications for such product candidate, that we develop;
• our ability to produce any product candidates we develop on a commercial scale;
• obtaining and maintaining patent, trademark and trade secret protection and regulatory exclusivity for our product candidate and otherwise protecting our rights in our intellectual property portfolio;
• maintaining compliance with regulatory requirements, including cGMP requirements;
• obtaining and maintaining coverage and adequate reimbursement by third-party payors, including government payors, for our products, if approved by the FDA;
• maintaining a continued acceptable safety, tolerability and efficacy profile of the products following approval; and
• maintaining and growing an organization of scientists and functional experts who can develop and commercialize our products and technology.
If we do not succeed with respect to one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize the product candidate we develop, which would materially harm our business. If we do not receive marketing approvals for any product candidate we develop, we may not be able to continue our operations. Even if regulatory approvals are obtained, we could experience significant delays or an inability to successfully commercialize our current and any future product candidates we develop, which would materially harm our business. If we are not able to generate sufficient revenue through the sale of any current or future product candidate, we may not be able to continue our business operations or achieve profitability.
The FDA regulatory approval process is lengthy and time-consuming and may lead to significant delays in the clinical development and regulatory approval of our product candidate.
The time required to obtain approval from the FDA is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of FDA. Any delay in obtaining FDA and/or other necessary regulatory approvals in the United States for any investigational new drug and failure to receive such approvals would have an adverse effect on the investigational new drug’s potential commercial success and on our business, prospects, financial condition, and results of operations.
We have not obtained regulatory approval for any product candidate. We have not previously submitted a BLA to the FDA. It is possible that none of our current or future product candidates will ever obtain regulatory approval from the FDA. The novel nature of our product candidate may create further challenges in obtaining regulatory approval. The regulatory approval pathway for our product candidate may be uncertain, complex, expensive, and lengthy, and approval may not be obtained. In addition, factors outside our control, such as government shutdowns, natural disasters, and public health emergencies, could disrupt business at the FDA, which could result in delays of reviews, approvals and communications with FDA related to our clinical trials and product candidates.
65
Our current and future product candidates could fail to receive regulatory approval for many reasons, including the following:
• the FDA may disagree with the design or implementation of our clinical trials;
• we may be unable to demonstrate to the satisfaction of FDA that a product candidate is safe, pure, and potent for its proposed indication;
• the results of clinical trials may not meet the level of statistical significance required by FDA for approval;
• we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks;
• the FDA may disagree with our interpretation of data from clinical trials or preclinical studies;
• the data collected from clinical trials of our product candidate may not be sufficient to support the submission of a BLA to the FDA to obtain regulatory approval in the United States; and
• FDA may find deficiencies with or fail to approve our manufacturing processes or facility or the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies.
The lengthy approval process as well as the unpredictability of clinical trial results may result in our failing to obtain regulatory approval to market any product candidate we develop, which would significantly harm our business, results of operations and prospects. FDA has substantial discretion in the approval process and in determining when or whether regulatory approval will be granted for any product candidate that we develop. Even if we believe the data collected from current or future clinical trials of our product candidate are promising, such data may not be sufficient to support approval by FDA.
Even if we obtain approval, FDA may approve any of our product candidate for fewer or more limited indications, or a more limited patient population, than we request; may grant approval contingent on the performance of costly post-approval clinical trials or other post-marketing requirements; or may approve a product candidate with labeling that does not include the claims we believe are necessary or desirable for the successful commercialization of such product candidates. Moreover, if we modify Apitox, we may have to either file a supplemental BLA with FDA or receive FDA approval for a comparability protocol or obtain other regulatory approval. These requirements may be costly and time-consuming, and FDA ultimately may not approve of such changes.
FDA may also change its policies, promulgate additional regulations, revise existing regulations, or take other actions that may prevent or delay approval of our future products under development on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain approvals, increase the costs of compliance or restrict our ability to maintain any marketing authorizations we may have obtained.
We may encounter substantial delays in our clinical trials or may not be able to conduct our trials on the timelines we expect.
Clinical testing is expensive, time consuming and subject to uncertainty. We cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. Even if these trials begin as planned, issues may arise that could suspend or terminate such clinical trials. A failure of one or more clinical studies can occur at any stage of testing, and our future clinical studies may not be successful. Events that may prevent successful or timely completion of clinical development include:
• inability to generate sufficient preclinical, toxicology or other in vivo or in vitro data to support the initiation of clinical trials;
• delays in sufficiently developing, characterizing or controlling a manufacturing process suitable for advanced clinical trials;
• delays in reaching a consensus with regulatory agencies on study design;
66
• delays in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical study sites;
• delays in obtaining required institutional review board (“IRB”), approval at each clinical study site;
• imposition of a temporary or permanent clinical hold by regulatory agencies for a number of reasons, including after review of an IND application or amendment, or equivalent application or amendment; as a result of a new safety finding that presents unreasonable risk to clinical trial participants; a negative finding from an inspection of our clinical study operations or study sites;
• developments on trials conducted by competitors for related technology that raises FDA concerns about risk to patients of the technology broadly; or if the FDA finds that the investigational protocol or plan is clearly deficient to meet its stated objectives;
• disruptions caused by any future pandemic may increase the likelihood that we encounter such difficulties or delays in initiating, enrolling, conducting or completing our planned clinical trials;
• delays in adding a sufficient number of trial sites;
• our ability to recruit suitable patients to participate in our clinical trials, which may be affected by, among other factors, patient eligibility and exclusion criteria defined in the protocol, the severity and difficulty of diagnosing the disease under investigation, the size of the patient population required for analysis of the trial’s primary endpoints, the proximity of patients to study sites, and the design of the trial;
• failure by our CROs, other third parties or us to adhere to clinical study requirements;
• failure to perform in accordance with the FDA’s good clinical practice, or GCP, requirements or applicable regulatory guidelines in other jurisdictions;
• transfer of manufacturing processes to any new CMO or our own manufacturing facilities or any other development or commercialization partner;
• patients dropping out of a study;
• occurrence of side effects associated with our product candidate that are viewed to outweigh their potential benefits;
• changes in regulatory requirements and guidance that require amending or submitting new clinical protocols;
• changes in the standard of care on which a clinical development plan was based, which may require new or additional trials;
• the cost of clinical trials being greater than we anticipate;
• clinical studies producing negative or inconclusive results, which may result in our deciding, or regulators requiring us, to conduct additional clinical studies or abandon product development programs;
• delays or failure to secure supply agreements with suitable raw material suppliers, or any failures by suppliers to meet our quantity or quality requirements for necessary raw materials; and
• delays in manufacturing, testing, releasing, validating or importing/exporting sufficient stable quantities of drug for use in clinical studies or the inability to do any of the foregoing.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenue. In addition, if we make manufacturing or formulation changes, we may be required to or we may elect to conduct additional studies to bridge our modified products to earlier versions. Clinical trial delays could also shorten any periods during which our products have patent protection and may allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our products and may harm our business and results of operations.
67
Our programs for which we intend to seek approval as biologics may face competition sooner than anticipated.
The Patient Protection and Affordable Act, as amended by the Healthcare and Education Reconciliation Act (the “ACA”), includes a subtitle called the Biologics Price Competition and Innovation Act of 2009 (“BPCIA”), which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, an application for a highly similar or “biosimilar” product may not be submitted to the FDA until four years following the date that the reference product was first approved by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first approved. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product.
We believe that any of our programs approved as biologics under a BLA should qualify for the 12-year period of exclusivity. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our programs to be reference products for competing products, potentially creating the opportunity for competition sooner than anticipated. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been the subject of recent litigation. Moreover, the extent to which a biosimilar, once approved, will be substituted for any reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing.
Because Apitox represents a novel approach to the treatment of symptoms for knee OA and potentially MS, there are many uncertainties regarding the development, market acceptance, third-party reimbursement coverage and commercial potential of our product candidate.
Because our candidate represents a novel approach to the treatment of the inflammation and pain management symptoms associated with knee OA and the potential treatment of MS, there are many uncertainties related to the development, marketing, reimbursement and the commercial potential for Apitox. There can be no assurance as to the length of the clinical trials, the number of patients the FDA will required to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of antibody products or that the design of or data generated in these trials will be acceptable to the FDA to support marketing approval.
In addition, the FDA may take longer than usual to come to a decision on any BLA that we submit and may ultimately determine that there is insufficient data, information or experience with our product candidates to support an approval decision. The FDA may also require that we conduct additional post-marketing studies or implement risk management programs, such as risk evaluation and mitigation strategies until more experience with our product candidate is obtained. Finally, after increased usage, we may find that our product candidate does not have the intended effect or have unanticipated side effects, potentially jeopardizing initial or continuing regulatory approval and commercial prospects.
Success in preclinical studies or earlier clinical trials may not be indicative of results in future clinical trials. Our product candidates may not have favorable results in later clinical trials, if any, or receive regulatory approval.
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate the same results or otherwise provide adequate data to demonstrate the efficacy and safety of a product candidate. Preclinical test and Phase I and Phase II clinical trials are primarily designed to test safety, to study pharmacokinetics and pharmacodynamics and to understand the side effects of product candidates at various doses and schedules. Success in preclinical or animal studies and early clinical trials does not ensure that later large-scale efficacy trials will be successful, nor does it predict final results. For example, we may be unable to identify suitable animal disease models for our product candidates, which could delay or frustrate our ability to progress clinical studies or obtain marketing approval. Our product candidates may fail to show the desired safety and efficacy in clinical development despite having progressed through preclinical and initial clinical trials.
Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks, delays. and failures in late-stage clinical trials even after achieving promising results in preclinical testing and earlier-stage clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit or prevent regulatory approval. In addition, we may experience regulatory delays or rejections as a result of many factors, including changes in regulatory policy during the period of our product candidate development.
68
Regulatory approval for any approved product is limited by the FDA to those specific indications and conditions for which clinical safety and efficacy have been demonstrated.
Any regulatory approval is limited to those specific diseases and indications for which a product is deemed to be safe and effective. If the FDA or any regulatory authority limits the scope of our indication, or if we are unable to obtain FDA approval for any desired future indications for our products, our ability to effectively market and sell our products may be reduced and our business may be adversely affected. Further, we are only permitted to promote our products for those indications that the FDA specifically approves and are restricted from making communications regarding uses not approved and described in the product’s labeling. If our promotional activities fail to comply with these regulations or guidelines, we may be subject to advisory or enforcement action by these authorities. In addition, our failure to follow FDA requirements or guidelines relating to promotion and advertising may cause the FDA to suspend or withdraw an approved product from the market, require a recall or institute fines, or could result in disgorgement of money, operating restrictions, injunctions or criminal prosecution, any of which could harm our business.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and management resources, we must focus on development programs and product candidates that we identify for specific indications. As such, we are currently primarily focused on the development of Apitox for the treatment of the inflammation and pain management symptoms associated with knee OA and MS. As a result, we may forego or delay pursuit of opportunities with other product candidates or other indications for these product candidates that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuation rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate.
If any potential future product candidate is approved and our CMO fails to produce the product in the volumes that we require on a timely basis, or to comply with stringent regulations applicable to pharmaceutical drug manufacturers, we may face delays in the commercialization of this product candidate or be unable to meet market demand and may lose potential revenues.
The manufacture of pharmaceutical products requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls, and the use of specialized processing equipment. Any termination or disruption of any current or future relationships relating to product development may materially harm our business and financial condition and frustrate any commercialization efforts for affected current or future product candidates.
Any current or future CMOs we engage must comply with strictly enforced federal, state and foreign regulations, including cGMP requirements enforced by the FDA through its establishment inspection program. Despite the existence of CMO agreements and shared cGMP responsibilities our contract CMO may ignore these contractual provisions, or otherwise fail to meet the minimum standards set forth in the cGMP regulations, resulting in manufacturing non-compliance. This may go unnoticed or uncorrected despite our best efforts to regulatory audit or confirm the CMOs regulatory responsibilities. Any failure to comply with applicable regulations may result in fines and civil penalties, suspension of production, suspension or delay in product approval, product seizure or recall, or withdrawal of product approval, and would limit the availability of our product. Any manufacturing defect or error discovered after products have been produced and distributed could result in even more significant consequences, including costly recalls, re-stocking costs, damage to our reputation and potential for product liability claims.
If the CMOs upon which we rely to manufacture any current products, and any potential product candidates we may in-license or acquire, fail to deliver the required commercial quantities on a timely basis at commercially reasonable prices, we would likely be unable to meet demand for our products and we would lose potential revenues.
69
Changes in methods of product candidate manufacturing or formulation may result in additional costs or delay.
As product candidates proceed through preclinical studies to late-stage clinical trials towards potential approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods and formulation, are altered along the way in an effort to optimize processes and results. Such changes carry the risk that they will not achieve these intended objectives. Any of these changes could cause our product candidate to perform differently and affect the results of planned clinical trials or other future clinical trials conducted with the materials manufactured using altered processes. Such changes may also require additional testing, FDA notification, or FDA approval. This could delay completion of clinical trials, require the conduct of bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of our product candidate, and jeopardize our ability to commence sales and generate revenue.
Our preclinical studies and clinical trials may fail to demonstrate substantial evidence of the safety and efficacy of our product candidates, or serious adverse or unacceptable side effects may be identified during the development of our product candidates, which could prevent, delay or limit the scope of regulatory approval of our product candidates, limit their commercialization, increase our costs or necessitate the abandonment or limitation of the development of some of our product candidates.
To obtain the requisite regulatory approvals for the commercial sale of our product candidates, we must demonstrate through lengthy, complex, and expensive preclinical testing and clinical trials that our product candidates are safe, pure and potent for use in each target indication. These trials are expensive and time consuming, and their outcomes are inherently uncertain. Failures can occur at any time during the development process. Preclinical studies and clinical trials often fail to demonstrate safety or efficacy of the product candidate studied for the target indication, and most product candidates that begin clinical trials are never approved.
We cannot commercialize product candidates in the United States without first obtaining regulatory approval from the FDA. Similarly, we cannot commercialize product candidates outside of the United States without obtaining regulatory approval from comparable foreign regulatory authorities. Before obtaining regulatory approvals for the commercial sale of Apitox, we must demonstrate through lengthy, complex and expensive preclinical and clinical trials that Apitox is both safe and effective for each targeted indication. Securing regulatory approval also requires the submission of information about the drug manufacturing process to, and inspection of manufacturing facilities by, the relevant regulatory authority. Further, a product candidate may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude its obtaining marketing approval. The FDA has substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical or other data. A product candidate could be delayed in receiving, or fail to receive, regulatory approval for many reasons.
Of the large number of drugs in development, only a small percentage successfully complete the FDA or foreign regulatory approval processes and are commercialized. The lengthy approval process as well as the unpredictability of future clinical trial results may result in us failing to obtain regulatory approval to market.
The regulatory approval processes of the FDA and other regulatory authorities are inherently unpredictable. If we are not able to obtain, or experience delays in obtaining, required regulatory approvals, we will not be able to commercialize Apitox in the United States.
If any current or future product candidates are associated with undesirable side effects, toxicities, or other negative characteristics, we may need to abandon such products’ development or limit development to more narrow uses or subpopulations. Such side effects may affect patient recruitment or the ability of enrolled patients to complete the trial and could result in potential product liability claims. Many compounds that show initial promise in early-stage testing are later found to cause side effects that prevent further development. If our clinical trials reveal severe or prevalent side effects, our trials could be suspended or terminated, we may be unable to recruit patients and enrolled patients may be unable to complete the trials, and the FDA or comparable foreign regulatory authorities could order issue a clinical hold or order us to cease further development or deny approval of the product candidate. Candidates may be harmed, which could significantly harm our business prospects.
70
All of our current and future products are subject to and will remain subject to substantial regulatory scrutiny even after receiving regulatory approval.
The preclinical and clinical development, testing, manufacture, safety, efficacy, labeling, storage, recordkeeping, and subsequent advertising, promotion, sale, marketing, and distribution, if approved, of our product candidate is subject to extensive regulation by the FDA and other regulatory authorities in the United States and elsewhere. These regulations also vary in important, meaningful ways from country to country. We are not permitted to market a potential drug in the United States until we receive approval of an NDA from the FDA for such drug. We have not received an NDA approval from the FDA for Apitox. There can be no guarantees with respect to our product candidate or future product candidates that clinical studies will adequately support an NDA, that the products will receive necessary regulatory approvals, or that they will prove to be commercially successful.
Further, any current or future product candidates we may license or acquire will be subject to ongoing regulatory and compliance requirements and oversight by the FDA and other regulatory authorities. These requirements include labeling, packaging, storage, advertising, promotion, record-keeping and submission of safety and other post-market information and reports, registration and listing requirements, cGMP requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and other licensed medical professionals and recordkeeping of the drug.
The Food and Drug Administration Amendments Act of 2007 granted significant expanded authority to the FDA, much of which was aimed at improving the safety of drug products before and after approval. The FDA may also impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product. The FDA closely regulates the post-approval marketing and promotion of drugs to ensure drugs are marketed only for the approved indications and in accordance with the provisions of the approved labeling.
The FDA imposes stringent restrictions on manufacturers’ communications regarding off-label use and if we do not market our products for only their approved indications, we may be subject to enforcement action for off-label marketing. While physicians and other healthcare providers may choose to prescribe drugs for uses that are not described in the product’s labeling and for uses that differ from those tested in clinical studies and approved by the regulatory authorities, our ability to promote the products is limited to those indications that are specifically approved by the FDA. These “off-label” uses are common across medical specialties and may constitute an appropriate treatment for some patients in varied circumstances. Regulatory authorities in the U.S. generally do not regulate the practice of medicine, including the clinical behavior of physicians and other healthcare providers in their choice of treatments. Regulatory authorities do, however, restrict communications by pharmaceutical companies on the subject of off-label use.
Violations of the Federal Food, Drug and Cosmetic Act relating to the promotion of prescription drugs may lead to investigations alleging violations of federal and state health care fraud and abuse laws, as well as state consumer protection laws.
In addition, later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may yield various results, including:
• restrictions on such products, operations, manufacturers or manufacturing processes;
• restrictions on the labeling or marketing of a product;
• restrictions on product distribution or use;
• requirements to conduct post-marketing studies or clinical trials;
• warning letters;
• withdrawal of the products from the market;
• refusal to approve pending applications or supplements to approved applications that we submit;
• recall of products;
• fines, restitution or disgorgement of profits;
• suspension or withdrawal of marketing or regulatory approvals;
71
• suspension of any ongoing clinical trials;
• denial of permits to import or export our products;
• product seizure; or
• injunctions or the imposition of civil or criminal penalties.
Public concern regarding the safety of any of our current or future drug products could delay or limit our ability to obtain regulatory approval, result in the inclusion of unfavorable information in our labeling, or require us to incur additional costs.
In light of widely publicized events concerning the safety risk of certain drug products, the FDA, members of Congress, the Government Accountability Office, medical professionals and the general public have raised concerns about potential drug safety issues. These events have resulted in the withdrawal of drug products, revisions to drug labeling that further limit use of the drug products, and the establishment of risk management programs. The increased attention to drug safety issues may result in a more cautious approach by the FDA in its review of data from our clinical trials. Data from clinical trials may receive greater scrutiny, particularly with respect to safety, which may make the FDA or other regulatory authorities more likely to require additional preclinical studies or clinical trials. If the FDA requires us to conduct additional preclinical studies or clinical trials prior to approving any other potential future product candidate, our ability to obtain such product candidate will be delayed. If the FDA requires us to provide additional clinical or preclinical data following the approval of any potential future product candidate, the indications for which such product candidate is approved may be limited or there may be specific warnings or limitations on dosing, and our efforts to commercialize potential future product candidate may be otherwise adversely impacted.
We face significant competition, and if our competitors develop and market technologies or products more rapidly than we do or that are more effective, safer or less expensive than the product we are commercializing or product candidates we develop, our commercial opportunities will be negatively impacted. Our product candidate will, if approved, also compete with existing branded, generic and off-label products.
The development and commercialization of new drug products is highly competitive. Numerous companies are engaged in developing products for the treatment of MS symptoms, which we expect will compete with Apitox. We face competition with respect to our product candidate from many different sources, including major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide and existing treatments. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than our products. Our competitors also may obtain FDA approval or other regulatory authority approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
In addition, our ability to compete may be affected in many cases by insurers or other third-party payers, particularly Medicare, seeking to encourage the use of generic products. Generic products are currently being used for certain of the indications that we are pursuing, and additional products are expected to become available on a generic basis over the coming years.
Many of the companies against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified sales, marketing scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
72
Even if we obtain regulatory approval of our products, the product may not gain market acceptance among regulators, advisory boards, physicians, patients, third-party payors and others in the medical community.
Even if our product receives marketing approval, it may fail to receive recommendations for use by regulators or advisory boards that recommend products, or gain market acceptance by physicians, patients, third-party payors and others in the medical community. If our product candidate does not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of any product, if approved for commercial sale, will depend on a number of factors, including but not limited to:
• the success of any potential clinic studies during the drug development process;
• physicians, hospitals, third-party payors and patients considering our products as safe and effective;
• the prevalence and severity of any side effects;
• product labeling or product insert requirements of the FDA or comparable foreign regulatory and advisory bodies;
• limitations or warnings contained in the labeling approved by the FDA or comparable foreign regulatory and advisory bodies;
• the timing of market introduction of our products as well as competitive products;
• the cost of treatment in relation to alternative treatments;
• the availability of coverage and adequate reimbursement and pricing by third-party payors, including government authorities;
• the willingness of patients to pay out-of-pocket in the absence of coverage and adequate reimbursement by third-party payors, including government authorities;
• relative convenience and ease of administration, including as compared to competitive products and alternative treatments; and
• the effectiveness of our sales and marketing efforts.
Our ability to effectively promote and sell our products and any other current or future product candidates we may develop, license or acquire in the marketplace will also depend on pricing and cost effectiveness, including our ability to produce a product at a competitive price and achieve acceptance of the product onto formularies, as well as our ability to obtain sufficient third-party coverage or reimbursement. Since many insurance plans are members of group purchasing organizations, which leverage the purchasing power of a group of entities to obtain discounts based on the collective buying power of the group, our ability to attract customers in the marketplace will also depend on our ability to effectively promote any current or future product candidates to group purchasing organizations. We will also need to demonstrate acceptable evidence of safety and efficacy, as well as relative convenience and ease of administration. Market acceptance could be further limited depending on the prevalence and severity of any expected or unexpected adverse side effects associated with our current or any future product candidates. If any current or future product candidates are approved but do not achieve an adequate level of acceptance by physicians, health care payors and patients, we may not generate sufficient revenue from these products, and we may not become or remain profitable. In addition, our efforts to educate the medical community and third-party payors on the benefits of any current or future product candidates may require significant resources and may never be successful.
Further, in both domestic and foreign markets, our any future product sales will depend in part upon the availability of coverage and reimbursement from third-party payors. Such third-party payors include government health programs such as Medicare, managed care providers, private health insurers and other organizations. We may need to conduct post-marketing studies in order to demonstrate the cost-effectiveness of any future products to the satisfaction of target customers and their third-party payors. Such studies might require us to commit a significant amount of management time and financial and other resources. Our current or future products might not ultimately be considered cost-effective. Adequate third-party coverage and reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
73
Our product candidates may cause undesirable side effects or have other properties that could halt their clinical development, prevent their regulatory approval, limit their commercial potential or result in significant negative consequences.
Adverse effects or other undesirable or unacceptable side effects caused by our product candidate could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authorities. Results of our clinical trials could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics. In such an event, our clinical trials could be suspended or terminated, and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidate. Such side effects could also affect trial recruitment or the ability of enrolled patients to complete the clinical trial or result in potential product liability claims. The data safety monitoring board may also suspend or terminate a clinical trial at any time on various grounds, including a finding that the research patients are being exposed to an unacceptable health risk. Treatment-related side effects could also affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims. If serious adverse or unacceptable side effects are identified during the development of any of any current or future product candidates, we may need to abandon such products’ development or limit development to more narrow uses or subpopulations. The need to show a high degree of safety and tolerability when dosing healthy individuals could result in rare and even spurious safety findings, negatively impacting a program prior to or after commercial launch. Adverse effects or other undesirable or unacceptable side effects caused by our product may cause us or the FDA to recall product or remove a product from the marketplace.
Any of these occurrences may harm our business, financial condition and prospects significantly.
Upon completion of Phase III clinical programs, we plan to develop a sales organization, and there is no assurance our marketing and sales organization will be successful.
If we are unable to successfully establish marketing and sales capabilities, we may not be able to generate product revenue. The development of an in-house marketing organization and sales force will require significant capital expenditures, management resources and time, and we will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train and retain marketing and sales personnel. There can be no assurance that our in-house sales and distribution capabilities will be successful.
If we are unable or decide not to successfully establish internal sales, marketing and distribution capabilities, we will pursue collaborative arrangements regarding the sales and marketing of our products; however, there can be no assurance that we will be able to establish or maintain such collaborative arrangements, or if we are able to do so, that they will have effective sales forces. Any revenue we receive will depend upon the efforts of such third parties, which may not be successful. We may have little or no control over the marketing and sales efforts of such third parties and our revenue from product sales may be lower than if we had commercialized our products ourselves. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our products.
We are highly dependent on our key personnel, and if we are not able to retain these members of our management team or recruit and retain highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive biotechnology and pharmaceutical industries depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. We are highly dependent on our management, scientific and medical personnel. There is currently a significant strain on our managerial, operational and financial resources. The loss of the services of our executive officers, other key employees and other scientific and medical advisors, and our inability to find suitable replacements could result in delays in product development and harm our business. We conduct substantially all of our operations at our facilities in the New York Area. This region is headquarters to many other biopharmaceutical companies and many academic and research institutions. Competition for skilled personnel in our market is intense and may limit our ability to hire and retain highly qualified personnel on acceptable terms or at all.
74
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the clinical testing of our product candidate and will face an even greater risk if we commercialize any products. For example, we may be sued if our products cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
• decreased demand for our products;
• injury to our reputation;
• withdrawal of clinical trial participants;
• initiation of investigations by regulators;
• costs to defend the related litigation;
• a diversion of management’s time and our resources;
• substantial monetary awards to trial participants or patients;
• product recalls, withdrawals or labeling, marketing or promotional restrictions;
• loss of revenue;
• exhaustion of any available insurance and our capital resources;
• the inability to commercialize any products; and
• a decline in our share price.
Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop, alone or with corporate collaborators. Our insurance policies may also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. Assuming we obtain clinical trial insurance for our clinical trials, we may have to pay amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Even if our agreements with any future corporate collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price.
The global credit and financial markets have experienced extreme volatility and disruptions in the past several years. Such volatility and disruptions have caused and may continue to cause severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. There can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, it may make any necessary debt or equity financing more difficult, more costly and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service providers, manufacturers and other partners may not survive an economic downturn, which could directly affect our ability to attain our operating goals on schedule and on budget.
75
Our business could be adversely affected by the effects of health epidemics, such as the COVID-19 pandemic, in regions where we or third parties on which we rely have significant manufacturing facilities, concentrations of potential clinical trial sites or other business operations. Any future pandemic could materially affect our operations, including at our headquarters in the New York area.
Health epidemics in regions where we have concentrations of potential clinical trial sites or other business operations could adversely affect our business, including by causing significant disruption in the operations of our CMO and other third parties upon whom we rely.
We or the third parties upon whom we depend on may be adversely affected by natural disasters or acts of war, such as the conflicts in Ukraine and Israel, and our business continuity and disaster recovery plans may not adequately protect us from any such serious disaster.
Any unplanned event, such as flood, fire, explosion, earthquake, extreme weather condition, medical epidemics, power shortage, telecommunication failure or other natural or man-made accidents or incidents that result in us being unable to undergo clinical trials, fully utilize our facilities, or the manufacturing facilities of our third-party CMOs, may have a material and adverse effect on our ability to operate our business, particularly on a daily basis, and have significant negative consequences on our financial and operating conditions. Loss of access to these facilities may result in increased costs, delays in the development of our product candidate or interruption of our business operations. Earthquakes or other natural disasters could further disrupt our operations and have a material and adverse effect on our business, financial condition, results of operations and prospects. If a natural disaster, power outage or other event were to occur that prevented us from using all or a significant portion of our headquarters, that damaged critical infrastructure, such as our research facilities or the manufacturing facilities of our third-party CMOs, or that otherwise disrupted operations, it may be difficult or, in certain cases, impossible, for us to continue our business for a substantial period of time.
Moreover, our CMOs may experience manufacturing difficulties due to resource constraints, governmental restrictions or as a result of labor disputes or unstable political environments. Supply chain issues, including those resulting from the COVID-19 pandemic and the ongoing military conflicts between Russian and Ukraine and Israel and surrounding areas and the attacks on marine vessels traversing the Red Sea, may affect our third-party vendors and cause delays. As of the date of this Information Statement, our CMOs and third-party vendors have not experienced manufacturing difficulties or delays as a result of the military conflicts in Ukraine and Israel.
As part of our risk management policy, we maintain insurance coverage at levels that we believe are appropriate for our business. However, in the event of an accident or incident at these facilities, we cannot assure you that the amounts of insurance will be sufficient to satisfy any damages and losses. If our facilities, or the manufacturing facilities of our third-party CMOs, are unable to operate because of an accident or incident or for any other reason, even for a short period of time, any or all of our research and development programs may be harmed and result in higher costs or adversely impact development of our product candidates.
Our employees, principal investigators, consultants and commercial partners may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants and commercial partners. Misconduct by these parties could include intentional failures, reckless and/or negligent conduct or unauthorized activities that violates (i) the laws and regulations of the FDA and other regulatory authorities, including those laws requiring the reporting of true, complete and accurate information to such authorities, (ii) manufacturing standards, (iii) federal and state data privacy, security, fraud and abuse and other healthcare laws and regulations in the United States, and (iv) laws that require the true, complete and accurate reporting of financial information or data.
In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct also could involve the improper use of individually identifiable information, including, without limitation, information obtained in the course of clinical trials, creating fraudulent data in our preclinical studies or clinical trials or illegal misappropriation of drug product, which could result in regulatory sanctions and cause serious harm to our reputation.
76
It is not always possible to identify and deter misconduct by employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. Additionally, we are subject to the risk that a person or government could allege such fraud or other misconduct, even if none occurred. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participating in government-funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws, contractual damages, reputational harm and the curtailment or restructuring of our operations, any of which could have a negative impact on our business, financial condition, results of operations and prospects. The shifting compliance environment and the need to build and maintain robust and expandable systems to comply with multiple jurisdictions with different compliance and/or reporting requirements increases the possibility that a healthcare company may run afoul of one or more of the requirements.
Risks Related to Our Reliance on Third Parties
We currently rely on third-party manufacturing and a single-source supplier to supply raw materials and components for, and manufacture, our product candidate. Our inability to have sufficient quantities of our product candidate manufactured, or our failure to comply with applicable regulatory requirements or to supply sufficient quantities at acceptable quality levels or prices, or at all, would materially and adversely affect our business.
Efficient and scalable manufacturing and supply is a vital component of our business strategy. We currently do not own or operate any manufacturing facilities. We have developed, in collaboration with third parties manufacturing processes that we believe can scale to address clinical and commercial supply. However, our assumptions as to our ability and our CMOs ability to produce our product candidate at the scale needed for clinical development and commercial demand may prove to be wrong. If we encounter problems in our manufacturing processes or in our ability to scale to address commercial drug supply, our business would be materially adversely affected.
The manufacturing process for a drug is subject to FDA or comparable foreign regulatory authority review. Our suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards, such as Current Good Manufacturing Practices, or cGMPs. If our CMOs cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or comparable foreign regulatory authorities, we may not be able to rely on their manufacturing facilities for the manufacture of elements of our product candidate. Moreover, we do not control the manufacturing process at our CMOs and are completely dependent on them for compliance with current regulatory requirements. In the event that any of our manufacturers fails to comply with such requirements or to perform its obligations in relation to quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may be forced to manufacture the materials ourselves or enter into an agreement with another third party, which we may not be able to do on reasonable terms, if at all.
We purchase the bee venom necessary to produce Apitox for our clinical trials from a single third-party supplier. There are a limited number of suppliers for bee venom, and we may need to assess alternate suppliers to prevent a possible disruption of the manufacture of Apitox for our clinical trials and, if approved, ultimately for commercial sale. Any termination of our supply agreement or significant delay in the supply of bee venom for the manufacture of Apitox for an ongoing clinical trial due to the need to replace a third-party supplier could considerably delay completion of our clinical trials, product testing and potential regulatory approval of Apitox.
Furthermore, if there is a disruption to our or our third-party suppliers’ relevant operations, including as a result of the COVID-19 pandemic, we will have no other means of producing Apitox until they restore the affected facilities or we or they procure alternative facilities. Additionally, any damage to or destruction of our or our third party or suppliers’ facilities or equipment may significantly impair our ability to manufacture Apitox on a timely basis.
77
If we change suppliers or manufacturers for commercial production, applicable regulatory agencies may require us to conduct additional studies or trials. If key suppliers or manufacturers are lost, or if the supply of the materials is diminished or discontinued, we may not be able to develop, manufacture and market our product candidate in a timely and competitive manner, or at all. Identifying and engaging an alternative supplier or manufacturer could result in delay, and we may not be able to find other acceptable suppliers or manufacturers on acceptable terms, or at all. Switching suppliers or manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines, which would impair our ability to meet our development objectives or generate revenues from the sale of Apitox, if approved.
We rely and will continue to rely on third parties to conduct our preclinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval of or commercialize our product candidate.
We currently do not have the ability to independently conduct preclinical or clinical studies that comply with the regulatory requirements known as Good Laboratory Practice and Good Clinical Practice (“GCP”). The FDA and regulatory authorities in other jurisdictions require us to comply with GCP requirements for conducting, monitoring, recording and reporting the results of clinical trials, in order to ensure that the data and results are scientifically credible and accurate and that the trial subjects are adequately informed of the potential risks of participating in clinical trials. We rely on independent investigators and collaborators, such as universities, medical institutions, CROs and strategic partners to conduct our preclinical and clinical trials under agreements with us.
We will need to negotiate budgets and contracts with CROs and study sites, which may result in delays to our development timelines and increased costs. We will rely heavily on these third parties over the course of our clinical trials, and we control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with applicable protocol and legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities. Our failure or any failure by these third parties to comply with these regulations or to recruit a sufficient number of patients may require us to repeat clinical trials, which would delay the regulatory approval process. Moreover, our business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
Any third parties conducting our preclinical studies and clinical trials will not be our employees and, except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our programs. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical trials or other drug development activities, which could affect their performance on our behalf. If these third parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to complete development of, obtain regulatory approval of or successfully commercialize our products. As a result, our financial results and the commercial prospects for our products would be harmed, our costs could increase and our ability to generate revenue could be delayed.
If any of our relationships with trial sites or any CRO that we may use in the future terminates, we may not be able to enter into arrangements with alternative trial sites or CROs or do so on commercially reasonable terms. Switching or adding third parties to conduct our clinical trials involves substantial cost and requires extensive management time and focus. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines.
78
If we or our third-party suppliers use hazardous, non-hazardous, biological or other materials in a manner that causes injury or violates applicable law, we may be liable for damages.
Our research and development activities involve the controlled use of potentially hazardous substances, including chemical and biological materials. We and our suppliers are subject to federal, state and local laws and regulations in the United States governing the use, manufacture, storage, handling and disposal of medical and hazardous materials. Although we believe that we and our suppliers’ procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, we and our suppliers cannot completely eliminate the risk of contamination or injury resulting from medical or hazardous materials. As a result of any such contamination or injury, we may incur liability or local, city, state or federal authorities may curtail the use of these materials and interrupt our business operations. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources. We do not have any insurance for liabilities arising from medical or hazardous materials. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental regulations may impair our research, development and production efforts, which could harm our business prospects, financial condition or results of operations.
We rely on honeybee colonies to supply our active pharmaceutical ingredient, or API, for Apitox, and if these colonies are damaged from pests, disease organisms or other phenomena, it could result in a negative impact on our business.
The mortality rate of honeybees has increased significantly over the last decade. Although the overall number of bee colonies in the United States. appears to be relatively stable, the increased mortality rate means beekeepers need to spend more time and money dividing their surviving colonies to create new ones to replace those lost. This could result in supply delays and cost increases for the bee venom we use to make Apitox. In addition, pests such as tracheal mites, Varroa mites and wax moths can either severely damage a bee colony or entirely wipe out a colony. American foulbrood is a lethal bacterial disease affecting bee larvae and pupae. Colonies may also be subject to “colony collapse disorder,” a phenomenological happening wherein the worker bees from a specific beehive suddenly vanish causing the hive to die off. Although our supplier employs preventative measures, any of these risks may negatively impact the bee hives and our ability to obtain the bee venom to make Apitox, which would adversely affect our business and prospects.
We may form or seek strategic alliances or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements.
We may form or seek strategic alliances, create joint ventures or collaborations or enter into additional licensing arrangements with third parties that we believe will complement or augment our discovery, development and commercialization efforts with respect to our products and any future products that we may seek to develop. Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners, and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our products because they may be deemed to be at too early of a stage of development for collaborative effort, and third parties may not view our products as having the requisite potential to demonstrate safety and efficacy. Any delays in entering into new strategic partnership agreements related to our products could delay the development and commercialization of our products in certain geographies for certain indications, which would harm our business prospects, financial condition and results of operations.
If we license products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture. We cannot be certain that, following a strategic transaction or license, we will achieve the results, revenue or specific net income that justifies such transaction.
79
Risks Related to Government Regulation
Our relationships with customers, physicians and third-party payors are subject, directly or indirectly, to federal and state healthcare fraud and abuse laws, health information privacy and security laws and other healthcare laws and regulations. If we or our employees, independent contractors, consultants, commercial partners and vendors violate these laws, we could face substantial penalties.
Healthcare providers, physicians and third-party payors in the United States and elsewhere will play a primary role in the recommendation and prescription of any products for which we have or obtain marketing approval. Our current and future arrangements with healthcare professionals, principal investigators, consultants, customers and third-party payors subject us to various federal and state fraud and abuse laws and other healthcare laws. These laws may constrain the business or financial arrangements and relationships through which we conduct our operations, including how we research, market, sell and distribute our products, if approved. Such laws include:
• the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or providing any remuneration (including any kickback, bribe or certain rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service, for which payment may be made, in whole or in part, under any U.S. federal healthcare program, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
• the U.S. federal civil and criminal false claims laws, including the civil False Claims Act, which can be enforced through civil whistleblower or qui tam actions, and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, to the U.S. federal government, claims for payment or approval that are false or fraudulent, knowingly making, using or causing to be made or used, a false record or statement material to a false or fraudulent claim, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the U.S. federal government. Pharmaceutical manufacturers can cause false claims to be presented to the U.S. federal government by engaging in impermissible marketing practices, such as the off-label promotion of a product for an indication for which it has not received FDA approval. In addition, the government may assert that a claim including items and services resulting from a violation of the U.S. federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act;
• the Health Insurance Portability and Accountability Act of 1996, or HIPAA, which prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for, healthcare benefits, items or services. Similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the healthcare fraud statute implemented under HIPAA or specific intent to violate it in order to have committed a violation;
• HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and its implementing regulations, which also impose certain obligations, including mandatory contractual terms, with respect to safeguarding the privacy and security of individually identifiable health information of covered entities subject to the rule, including health plans, healthcare clearinghouses and certain healthcare providers as well as their business associates, independent contractors of a covered entity that perform certain services involving the use or disclosure of individually identifiable health information for or on their behalf;
• the Federal Food Drug or Cosmetic Act, which prohibits, among other things, the adulteration or misbranding of drugs, biologics and medical devices;
80
• the U.S. Physician Payments Sunshine Act and its implementing regulations, which require certain manufacturers of drugs, devices, biologics and medical supplies that are reimbursable under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the Centers for Medicare and Medicaid Services, or CMS, information related to certain payments and other transfers of value to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by the physicians described above and their immediate family members; and
• analogous U.S. state laws and regulations, including: state anti-kickback and false claims laws, which may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by any third-party payor, including private insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws and regulations that require drug manufacturers to file reports relating to pricing and marketing information, which require tracking gifts and other remuneration and items of value provided to healthcare professionals and entities; state and local laws requiring the registration of pharmaceutical sales representatives; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
We may also be subject to other laws, such as the U.S. Foreign Corrupt Practices Act of 1977, as amended, which prohibit, among other things, U.S. companies and their employees and agents from authorizing, promising, offering or providing, directly or indirectly, corrupt or improper payments or anything else of value to foreign government officials, employees of public international organizations and foreign government owned or affiliated entities, candidates for foreign political office and foreign political parties or officials thereof, as well as federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers.
Ensuring that our internal operations and business arrangements with third parties comply with applicable healthcare laws and regulations will likely be costly. It is possible that governmental authorities will conclude that our business practices, including our relationships with physicians and other healthcare providers, some of whom are compensated in the form of stock options for consulting services provided, may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participating in government-funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws, contractual damages, reputational harm and the curtailment or restructuring of our operations.
Even if resolved in our favor, litigation or other legal proceedings relating to healthcare laws and regulations may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development, manufacturing, sales, marketing or distribution activities. Uncertainties resulting from the initiation and continuation of litigation or other proceedings relating to applicable healthcare laws and regulations could have an adverse effect on our ability to compete in the marketplace. In addition, if the physicians or other providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, they may be subject to significant criminal, civil or administrative sanctions, including exclusions from government-funded healthcare programs.
81
Changes in funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent new products and services from being developed or commercialized in a timely manner, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, their ability to hire and retain key personnel and accept the payment of user fees and statutory, regulatory and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
Of note, in response to the global COVID-19 pandemic, the FDA adopted a risk-based system for the conduct of inspections of manufacturing facilities and began conducting voluntary remote interactive evaluations of certain drug manufacturing facilities and clinical research sites where an in-person inspection would not be prioritized, deemed mission-critical, or where direct inspection is otherwise limited by travel restrictions, but where the FDA determines that remote evaluation would still be appropriate. Regulatory authorities inside and outside the United States may adopt similar restrictions or other policy measures in response to any future pandemic. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
We are subject to increasingly stringent and rapidly changing laws and regulations related to privacy and data security. The restrictions and costs imposed by these requirements, or our actual or perceived failure to comply with them, could harm our reputation, subject us to significant fines and liability, and adversely affect our business.
We are subject to or affected by numerous evolving federal, state and foreign laws and regulations, as well as policies, contracts and other obligations governing the collection, use, disclosure, retention, and security of personal data. The global data protection landscape is rapidly evolving, and implementation standards and enforcement practices are likely to remain uncertain for the foreseeable future. This landscape may create uncertainty in our business, result in liability or impose additional costs on us. These laws and regulations are subject to differing interpretations and may be inconsistent among jurisdictions, and guidance on implementation and compliance practices are often updated or otherwise revised. The cost of compliance with these laws and regulations is high and is likely to increase in the future. Our failure or perceived failure to comply with these laws and regulations could result in negative publicity, diversion of management time and effort, an inability to process personal data or to operate in certain jurisdictions, restrictions on our operations and legal action against us by governmental entities or others. In many jurisdictions, enforcement actions and consequences for noncompliance are rising.
For example, HIPAA, as amended by HITECH, imposes requirements relating to the privacy and security of individually identifiable health information on health plans, healthcare clearinghouses and certain healthcare providers, and their respective contractors and their covered subcontractors that perform services for them involving individually identifiable health information. Additionally, certain states have adopted healthcare privacy and security laws and regulations comparable to HIPAA, some of which may be more stringent than HIPAA. In the event we fail to properly maintain the privacy and security of individually identifiable health information governed by HIPAA or comparable state laws, and significant or we are responsible for an unauthorized disclosure or security breach of such information, we could be subject to enforcement action under HIPAA or comparable state laws, civil and criminal penalties, and fines.
82
Domestic privacy and data security laws beyond HIPAA and other healthcare privacy laws are also changing rapidly and becoming more complex. For example, California recently enacted the CCPA, which took effect on January 1, 2020. The CCPA gives California residents expanded rights to access and delete their personal information, opt-out of certain personal information sharing, and receive detailed information about how their personal information is used, among others. The CCPA also requires covered businesses to provide detailed privacy notices to California residents and respond to requests from California residents to exercise their rights under the CCPA to access, delete and opt-out of certain sharing of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Although there are limited exemptions for clinical trial data, the CCPA may increase our compliance costs and potential liability. Some observers have noted that the CCPA could mark the beginning of a trend toward more stringent privacy legislation in the U.S. As of January 1, 2023, consumers have new rights in addition to those above, such as (i) the right to correct inaccurate personal information that a business has about them; and (ii) the right to limit the use and disclosure of sensitive personal information collected about them. The CPRA will, among other things, give California residents the ability to limit use of certain sensitive personal information, further restrict the use of cross-contextual advertising, establish restrictions on the retention of personal information, expand the types of data breaches subject to the CCPA’s private right of action, provide for increased penalties for CPRA violations concerning California residents under the age of 16, and establish a new California Privacy Protection Agency to implement and enforce the new law. Although there are limited exemptions for clinical trial data under the CCPA, the CCPA and other similar laws could impact our business activities depending on how it is interpreted.
If our product candidates are approved for marketing and are found to have been improperly promoted for off-label uses, or if physicians misuse our products or use our products off-label, we may become subject to prohibitions on the sale or marketing of our products, product liability claims and significant fines, penalties and sanctions, and our brand and reputation could be harmed.
If our approved product, or any of our product candidates that are approved, are found to have been improperly promoted for off-label uses of those products, we may become subject to significant liability. The FDA and other regulatory agencies strictly regulate the promotional claims that may be made about approved prescription drug products. In particular, while the FDA permits the dissemination of truthful and non-misleading information about an approved product, a manufacturer may not promote a product for uses that are not approved by the FDA. If we are found to have promoted such off-label uses, we may become subject to significant liability. The federal government has levied large civil and criminal fines against companies for alleged improper promotion of regulated products for off-label uses and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees, corporate integrity agreements or permanent injunctions under which specified promotional conduct must be changed or curtailed. If we cannot successfully manage the promotion of our product candidate, if approved, we could become subject to significant liability, which would materially adversely affect our business and financial condition.
We are subject to new legislation, regulatory proposals and managed care initiatives that may increase our costs of compliance and adversely affect our ability to market our products, obtain collaborators and raise capital.
In the United States and certain foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the “ACA”), was signed into law, which substantially changed the way healthcare is financed by both governmental and private insurers in the United States. By way of example, the ACA: increased the minimum level of Medicaid rebates payable by manufacturers of brand name drugs from 15.1% to 23.1%; required collection of rebates for drugs paid by Medicaid managed care organizations; imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell certain “branded prescription drugs” to specified federal government programs; implemented a new methodology under which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted, or injected; expanded the eligibility criteria for Medicaid programs; created a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and established a Center for Medicare and Medicaid Innovation (“CMMI”) at the Centers for Medicare & Medicaid Services (“CMS”), to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending.
83
Since its enactment, there have been executive, judicial and Congressional challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. President Trump signed several Executive Orders and other directives designed to delay the implementation of certain provisions of the ACA or otherwise circumvent some of the requirements for health insurance mandated by the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed comprehensive repeal legislation, several bills affecting the implementation of certain taxes under the ACA have been enacted. For example, in 2017, Congress enacted the Tax Cuts and Jobs Act, which eliminated the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year, a process that is commonly referred to as the “individual mandate.” In addition, the Further Consolidated Appropriations Act, 2020 permanently eliminated, effective January 1, 2020, the ACA-mandated “Cadillac” tax on high-cost employer-sponsored health coverage and medical device tax and, effective January 1, 2021, it also eliminated the health insurer tax. On December 14, 2018, the U.S. District Court for the Northern District of Texas ruled that the individual mandate is a critical and inseverable feature of the ACA, and therefore, because it was repealed as part of the Tax Act, the remaining provisions of the ACA are invalid as well. On December 18, 2019, the U.S. Court of Appeals for the Fifth Circuit ruled that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the ACA are invalid as well. On March 2, 2020, the U.S. Supreme Court reversed the Fifth Circuit’s ruling, holding that the challengers lacked standing to sue and otherwise abstaining from reaching the merits of the case. There may be other efforts to challenge, repeal, or replace the ACA. We are continuing to monitor any changes to the ACA that, in turn, may potentially impact our business in the future.
Former President Biden signed an Executive Order on Strengthening Medicaid and the Affordable Care Act, stating his administration’s intentions to reverse the actions of his predecessor and strengthen the ACA. As part of this Executive Order, the Department of Health and Human Services, United States Treasury, and the Department of Labor are directed to review all existing regulations, orders, guidance documents, policies and agency actions to consider if they are consistent with ensuring coverage under the ACA and making high-quality healthcare affordable and accessible to Americans. We are unable to predict the likelihood of changes to the ACA or other healthcare laws which may negatively impact our profitability.
Former President Biden intended, as his predecessor did, to take action against drug prices which are considered “high.” Such measures could be addressed in a legislative package later in 2021 or with the reauthorization of the Prescription Drug User Fee Act, or PDUFA, in 2022 as part of a package bill. Drug pricing continues to be a subject of debate at the executive and legislative levels of U.S. government, and we expect to see legislation focusing on this in the coming year. The American Rescue Plan Act of 2021 signed into law by President Biden on March 14, 2021 includes a provision that eliminated the statutory cap on rebates drug manufacturers pay to Medicaid beginning in January 2024. With the elimination of the rebate cap, manufacturers may be required to compensate states in an amount greater than what the state Medicaid programs pay for the drug.
Other legislative changes have been proposed and adopted since the ACA was enacted. These changes include aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, effective April 1, 2013, which, due to subsequent legislative amendments, will stay in effect through 2030 with the exception of a temporary suspension implemented under various COVID-19 relief legislation from May 1, 2020 through December 31, 2021, unless additional congressional action is taken. Moreover, there has recently been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed and enacted legislation designed, among other things, to bring more transparency to product pricing, to review the relationship between pricing and manufacturer patient assistance programs, and to reform government program reimbursement methodologies for pharmaceutical products. The Prescription Drug Pricing Reduction Act, or PDPRA, which was introduced in Congress in 2019, and again in 2020, proposed to, among other things, penalize pharmaceutical manufacturers for raising prices on drugs covered by Medicare Parts B and D faster than the rate of inflation, cap out-of-pocket expenses for Medicare Part D beneficiaries, and proposes several changes to how drugs are reimbursed in Medicare Part B. A similar drug pricing bill, the Elijah E. Cummings Lower Drug Costs Now Act proposes to enable direct price negotiations by the federal government for certain drugs (with the maximum price paid by Medicare capped based on an international index), requires manufacturers to offer these negotiated prices to other payors, and restricts manufacturers from raising prices on drugs covered by Medicare Parts B and D. This Act passed in the House of Representatives when it was introduced in 2019, and it has been introduced again in the 2021 term. We cannot predict whether any proposed legislation will become law and the effect of these possible changes on our business cannot be predicted at this time.
84
Further, the Centers for Medicare & Medicaid Services (“CMS”) has significant regulatory authority to promulgate regulations and impose other compliance requirements that may increase our compliance costs and impact our ability to attain profitability and market our product candidate. CMS sets coverage and reimbursement rates for Medicare and oversees the implementation of Medicaid at the state level. CMS could modify or impose coverage restrictions or modify reimbursement rates on our product candidate in a manner that could adversely impact our business. For example, on January 8, 2021, CMS approved Tennessee’s Medicaid section 1115 demonstration application, granting the state the unprecedented ability to implement a closed drug formulary without foregoing the state’s entitlement to rebates under the Medicaid Drug Rebate Program. Implementation of a closed formulary could mean that our products could be excluded from coverage under Medicaid. It is unclear whether the Biden Administration will reverse or modify Tennessee’s section 1115 demonstration approval.
Within CMS, CMMI, as established by the ACA, has broad authority to design, implement, and test new health care payment models that could potentially lower health care spending while maintaining quality or increase quality without increasing spending. CMMI has considered implementing models that could have a significant adverse effect on our business. For example, on November 27, 2020, CMMI finalized a mandatory Medicare Part B drug payment model that would have aligned payment for drugs with international reference prices, entitled the Most Favored Nation (MFN) Model. The MFN Model was enjoined by a Federal court on December 28, 2020 for failure to comply with rulemaking procedural requirements. It is unclear whether the Biden Administration will propose and implement the same or a similar model in future rulemaking, and we cannot predict how future regulatory actions by CMMI or any other component of CMS may impact our business.
These and other healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for our current or future product candidates. Any reduction in reimbursement from Medicare or other government healthcare programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for drugs. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of any current or future product candidates, if any, may be. In addition, increased Congressional scrutiny of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
Any product candidates for which we intend to seek approval as biologic products may face biosimilar competition sooner than anticipated.
If we are successful in achieving regulatory approval to commercialize any biologic product candidate that we develop, it may face competition from biosimilar products. In the United States, our product candidates are regulated by the FDA as biologic products subject to approval under the BLA pathway. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the ACA, includes a subtitle called the Biologics Price Competition and Innovation Act of 2009, or BPCIA, which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the data the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed by the FDA. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The law is complex and is still being interpreted and implemented by the FDA. As a result, its ultimate impact, implementation and meaning are subject to uncertainty. While it is uncertain when such processes intend to be implemented, BPCIA may be fully adopted by the FDA, any such processes could have an adverse effect on the future commercial prospects for our biological products. There is a risk that any of our product candidates approved as a biological product under a BLA would not qualify for the 12-year period of exclusivity or that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for generic competition sooner than anticipated.
85
For example, in May 2021, the Biden administration expressed support for waiving intellectual property protections for COVID-19 vaccines amid concerns about vaccines access in foreign nations. Such waiver, if implemented, could extend to our product candidates. Other aspects of the BPCIA, some of which may impact the BPCIA exclusivity provisions, have also been subject of recent litigation. Moreover, the extent to which a biosimilar, once approved, will be substituted for any of our reference products in a way that is similar to traditional generic substitution for non-biological products is not yet clear, and will depend on a number of marketplace and regulatory factors that are still developing. If competitors are able to obtain marketing approval for biosimilars referencing our candidates, if approved, our products may become subject to competition from such biosimilars, with the attendant competitive pressure and potential adverse consequences.
Our business activities will be subject to the Foreign Corrupt Practices Act, or FCPA, and similar anti-bribery and anti-corruption laws.
As we expand our business activities outside of the United States, including our clinical trial efforts, we will be subject to the FCPA and similar anti-bribery or anti-corruption laws, regulations or rules of other countries in which we intend to operate. The FCPA generally prohibits offering, promising, giving or authorizing others to give anything of value, either directly or indirectly, to a non-United States government official in order to influence official action, or otherwise obtain or retain business. The FCPA also required public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls. Our business is heavily regulated and therefore may involve significant interaction with public officials, including officials of non-United States governments. Additionally, in many other countries, the healthcare providers who prescribe pharmaceuticals are employed by their government under the FCPA. Recently, the SEC and Department of Justice have increased their FCPA enforcement activities with respect to biotechnology and pharmaceutical companies. There is no certainty that all of our employees, agents, suppliers, manufacturers, contractors or collaborators, of those of our affiliates, will comply with all applicable laws and regulations, particularly given the high level of complexity of these laws. Violations of these laws and regulations could result in fines, criminal sanctions against us, our officers, or our employees, the closing down of facilities, including those of our suppliers and manufacturers, requirements to obtain export licenses, cessation of business activities in sanctioned countries, implementation of compliance programs and prohibitions on the conduct of our business. Any such violations could include prohibitions on our ability to offer our products in one or more countries as well as difficulties in manufacturing or continuing to develop our products, and could materially damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees, and our business, prospects, operating results, and financial condition.
Risks Related to Our Intellectual Property
We rely principally on trade secrets and other forms of non-patent intellectual property protection, which are difficult to protect.
Our API is a natural, non-synthetic compound that is not patentable, so we rely on trade secrets to protect our rights to Apitox, particularly the method and process of manufacturing Apitox. Trade secrets are difficult to protect, and we have limited control over the protection of trade secrets used by our collaborators and suppliers. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors and other advisors may unintentionally or willfully disclose our information to competitors. We face the risk of potential unauthorized disclosure or misappropriation of our intellectual property, which may reduce our trade secret protection and allow our potential competitors to access and exploit our proprietary technology. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. We would expect any trade secret dispute to be governed by federal law, and in particular the Defend Trade Secrets Act (“DTSA”) of 2016. However, in the event we are not able to utilize the DTSA, we would then be limited to resolving such dispute in state court. State trade secret laws in the United States vary, and state courts are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how. If our confidential or proprietary information is divulged to or acquired by third parties, including our competitors, our competitive position in the marketplace will be harmed and our ability to successfully penetrate our target markets could be severely compromised.
86
Our ability to compete depends in part on our ability to secure and maintain proprietary rights to our products.
Apimeds Korea does not have any patent protection for Apitoxin. There could be several competitive products available in the marketplace possessing similar qualities.
Apimeds Korea’s patents related to Apitoxin expired in early 2023. If Apimeds does decide to apply for patent protection, there can be no assurance that any patent issued Apimeds Korea, and licensed to us, will provide competitive advantages or will not be challenged by third parties. Furthermore, there can be no assurance that others will not independently develop similar products or design around Apimeds Korea’s previous patents. Any of the foregoing activities could have a material adverse effect on the Company. Moreover, enforcement of any patent or license rights may require substantial litigation costs.
Our success depends in part on not only our ability, but that of Apimeds Korea’s to protect the intellectual property, including our trade secrets, which can be difficult and costly and is not assured.
Our success depends in part on our ability, and that of Apimeds Korea, to obtain and maintain trade secret and trademark protection of our rights to Apitox, as well as successfully defending the intellectual property against third-party challenges. Our ability to stop unauthorized third parties from misusing our trade secrets by making or using Apitox is dependent upon the extent to which we have rights under trade secrets that cover these activities.
We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, consulting agreements or other similar agreements with our advisors, employees, third-party contractors and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of these parties to use or disclose our confidential information, including our trade secrets. The degree of future protection for our proprietary rights is uncertain because legal means afford only limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage. The intellectual property positions of biopharmaceutical companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. We cannot accurately predict future changes in the interpretation of intellectual property laws or changes to intellectual property laws which might be enacted into law. Those changes may materially affect our ability to protect our trade secrets.
Despite our efforts to protect our trade secrets, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others or are disclosed or used in violation of these agreements. Moreover, we cannot guarantee that we have entered into such agreements with each party that may have or have had access to our confidential information or proprietary technology and processes. Monitoring unauthorized uses and disclosures is difficult, and we do not know whether the steps we have taken to protect our proprietary technologies will be effective. If any of the collaborators, scientific advisors, employees, contractors and consultants who are parties to these agreements breaches or violates the terms of any of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets as a result. Moreover, if confidential information that is licensed or disclosed to us by our partners, collaborators or others is inadvertently disclosed or subject to a breach or violation, we may be exposed to liability to the owner of that confidential information. Failure to protect our trade secrets, for any reason (or third-party claims against our trade secrets or proprietary rights, or our involvement in disputes over our trade secrets or proprietary rights, including involvement in litigation), could have a substantial negative effect on our results of operations and financial condition.
We do not own the Apitox trademark but may use the trademark pursuant to the terms of the Business Agreement with Apimeds Korea.
We do not own the trademark that we use in our business and may be unable to protect this intellectual property against infringement from third parties. We are party to the Business Agreement with Apimeds Korea pursuant to which Apimeds Korea granted us an exclusive, sublicensable, royalty-bearing license to utilize all prior clinical development data associated with Apitoxin, Apitox, and all related names, advance clinical research, develop, manufacture and commercialize and sell Apitox in the United States. The Business Agreement can be terminated by mutual written agreement by the parties and will automatically terminate upon the bankruptcy or dissolution of the Company. In the event that the Business Agreement is terminated, we will be required to, among other things, change the name of our product candidate.
87
Any of these events could disrupt our recognition in the marketplace, damage any goodwill we may have generated, and otherwise have a material adverse effect on us. Furthermore, since we license the use of the name “Apitox” from Apimeds Korea, we are dependent on Apimeds Korea to defend against trademark infringement claims. Apimeds Korea’s efforts to enforce or protect our rights related to the trademark “Apitox” may be ineffective and could result in substantial costs and diversion of resources and could adversely impact our financial condition or results of operations.
If our future trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
We do not own any trademarks or tradenames, as we license the use of the name “Apitox” from Apimeds Korea pursuant to the Business Combination Agreement; however, in the future if we are to register and trademarks or tradenames, any registered trademarks or trade names may be challenged, circumvented, or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely impact our financial condition or results of operations.
We may become involved in lawsuits to protect our intellectual property rights, which could be expensive, time consuming and unsuccessful.
Competitors may infringe or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, we may be required to file legal claims, which can be expensive and time-consuming. An adverse result in any litigation or defense proceedings could put our intellectual property at risk of being invalidated or interpreted narrowly. The initiation of a claim against a third party may also cause the third party to bring counter claims against us such as claims asserting that our intellectual property rights are invalid or unenforceable. The outcome following legal assertions of invalidity and unenforceability is unpredictable. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States. Our business could be harmed if in litigation the prevailing party does not offer us a license on commercially reasonable terms. Any litigation or other proceedings to enforce our intellectual property rights may fail, and even if successful, may result in substantial costs and distract our management and other employees.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have an adverse effect on the price of our common shares.
Any trademarks we may obtain may be infringed or successfully challenged, resulting in harm to our business.
We expect to rely on trademarks as one means to distinguish our products from those approved for marketing from the products of our competitors. We intend to file a United States trademark application for “Apitox” but have not yet begun the process of applying to register any trademarks for our current product candidate or any future products. Once we select trademarks and apply to register them, our trademark applications may not be approved. Third parties may oppose our trademark applications or otherwise challenge our use of the trademarks. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition and could require us to devote resources to advertising and marketing new brands. Our competitors may infringe our trademarks, and we may not have adequate resources to enforce our trademarks. In addition, any proprietary name we propose to use with our current or any other product candidates in the United States must be approved by the FDA,
88
regardless of whether we have registered it, or applied to register it, as a trademark. The FDA typically conducts a review of proposed product names, including an evaluation of the potential for confusion with other product names. If the FDA objects to any of our proposed proprietary product names, we may be required to expend significant additional resources in an effort to identify a suitable proprietary product name that would qualify under applicable trademark laws, not infringe the existing rights of third parties and be acceptable to the FDA.
Furthermore, pursuant to the Business Agreement, Apimeds Korea granted to the Company a sublicensable, royalty-bearing license to utilize all prior clinical development data associated with Apitoxin, Apitox, and all related names, and to advance clinical research, develop, manufacture and commercialize and sell Apitox in the United States. The Company does not own international rights to the name “Apitox,” and the Company has an exclusive license to use the name in the United States pursuant to the Business Agreement. The name “Apitox” may be used in different countries with respect to products that are not ours. We are aware of a Spanish company called PrismaNatural, offering an over-the-counter topical ointment product called “Apitox” that can be purchased in the United States. This may create confusion with respect to our product candidate and our business may be adversely affected.
As mentioned above, we are dependent on Apimeds Korea to defend against trademark infringement claims because we license the use of the name “Apitox” pursuant to the Business Agreement. Notwithstanding Apimeds Korea’s efforts, there can be no assurance that its efforts to protect its trademarks will be successful, even if Apimeds Korea intends to maintain and keep current all of its trademark registrations and to pay all applicable renewal fees as they become due. The right of a trademark owner to use its trademarks is based on a number of factors, including their first use in commerce, and trademark owners can lose trademark rights despite trademark registration and payment of renewal fees. We therefore believe that these proprietary rights, licensed to us, have been and will continue to be important in enabling us to compete and if for any reason Apimeds Korea is unable to maintain its trademark that it licenses to us, our business could be materially and negatively affected. Nor can there be any assurance that third-parties will not assert claims against us for infringement of their intellectual proprietary rights. If an infringement claim is asserted, we may be required to obtain a license of such rights, pay royalties on a retrospective or prospective basis, or terminate our development, manufacturing and marketing of our infringing products. Litigation with respect to such matters could result in substantial costs and diversion of management and other resources and could have a material adverse effect on our business, financial condition, or operating results.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we expect to rely on third parties to manufacture Apitox and any future candidates, and we expect to collaborate with third parties on the development of our current and future therapeutics, we must, at times, share trade secrets with them. We also may conduct joint research and development programs that may require us to share trade secrets under the terms of our research and development partnerships or similar agreements. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, consulting agreements or other similar agreements with our advisors, employees, third-party contractors and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, including our trade secrets. Despite the contractual provisions employed when working with third parties, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by our competitors, are inadvertently incorporated into the technology of others or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, a competitor’s discovery of our trade secrets or other unauthorized use or disclosure would impair our competitive position and may have an adverse effect on our business and results of operations. Further, disputes may arise under these agreements regarding inventorship or ownership of proprietary information generated during research and development.
In addition, these agreements typically restrict the ability of our advisors, employees, third-party contractors and consultants to publish data potentially relating to our trade secrets, although our agreements may contain certain limited publication rights. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of our agreements with third parties, independent development or publication of information by any of our third-party collaborators. A competitor’s discovery of our trade secrets would impair our competitive position and have an adverse impact on our business.
89
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of their former employers or other third parties.
We employ individuals who were previously employed at other biotechnology or pharmaceutical companies. Although we seek to protect our ownership of intellectual property rights by ensuring that our agreements with our employees, collaborators and other third parties with whom we do business include provisions requiring such parties to assign rights in inventions to us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of our employees’ former employers or other third parties. We may also be subject to claims that former employers or other third parties have an ownership interest in our patents. Litigation may be necessary to defend against these claims. There is no guarantee of success in defending these claims, and if we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Even if we are successful, litigation could result in substantial cost and be a distraction to our management and other employees.
Risks Related to the Conversion of Preferred Stock Issued as Merger Consideration
The conversion of the shares of Preferred Stock into shares of Common Stock will significantly dilute existing holders of Common Stock.
The conversion of the Preferred Stock issued pursuant to the Merger Agreement into shares of Common Stock will result in the issuance of a substantial number of additional shares of Common Stock. This issuance will dilute the ownership interests and voting power of existing holders of Common Stock and may adversely affect the market price of the Common Stock.
The conversion may materially change the Company’s capital structure and stockholder base.
Following the Preferred Stock Conversion, the Company’s capital structure will change significantly, including an increase in the number of outstanding shares of Common Stock. As a result, former holders of Preferred Stock may hold a significant percentage of the Company’s outstanding Common Stock and may be able to exert substantial influence over matters requiring stockholder approval.
The market price of the Common Stock may decline as a result of the Preferred Stock Conversion.
The issuance of a large number of shares of Common Stock upon the Preferred Stock Conversion could result in increased selling pressure in the public market, which could cause the trading price of the Common Stock to decline, potentially significantly.
Holders of the Preferred Stock may have interests that differ from those of existing holders of the Common Stock.
The Preferred Stock was issued pursuant to the Merger Agreement as part of the Merger Consideration and has rights and preferences that differ from those of the Common Stock, including conversion rights. As a result, the interests of the holders of the Preferred Stock in connection with the timing and the terms of the conversion may differ from, or conflict with, the interests of existing holders of Common Stock.
Risks Related to Ownership of Our Common Stock
The price of our stock may be volatile, and you could lose all or part of your investment.
The trading price of our common stock could be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume. In addition to the factors discussed in this “Risk Factors” section and elsewhere in this Information Statement, these factors include:
• the commencement, enrollment, progress or results of our planned or future preclinical studies or clinical trials of our products and those of our competitors;
• delays or terminations of clinical trials;
90
• regulatory or legal developments in the United States;
• announcements by our competitors of new product candidates or technologies, or the results of clinical trials or regulatory decisions;
• the recruitment or departure or key personnel;
• developments or disputes concerning patent applications, issued patents or other proprietary rights;
• the level of expenses related to our products or preclinical and clinical development programs;
• the results of our efforts to develop additional products;
• unanticipated serious safety concerns related to the use of any product candidate;
• actual or anticipated changes in estimates as to financial results, development timelines or recommendations or reports by securities analysts;
• the level of expenses and capital investment related to manufacturing out products;
• our inability to obtain or delays in obtaining adequate supply for any approved products candidate;
• significant lawsuits, including patent or stockholder litigation;
• variations in our financial results or those of companies perceived to be similar to us;
• changes in the structure of healthcare payment systems, including coverage and adequate reimbursement for any approved medicines;
• publication of research reports about us or our industry or positive or negative recommendations or withdrawal of research coverage by securities analysts;
• overall performance of the equity markets;
• general economic, political and market conditions and overall fluctuations in the financial markets in the United States; and
• investors’ general perception of us and our business.
In addition, the stock market in general, the NYSE American and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies, which has resulted in decreased stock prices for many companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. If the market price of our common stock after this offering does not exceed the initial public offering price, you may not realize any return on your investment in us and may lose some or all of your investment. In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation, if instituted, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition.
Our financial condition and results of operations may fluctuate from quarter to quarter and year to year, which makes them difficult to predict.
We expect our financial condition and results of operations to fluctuate from quarter to quarter and year to year due to a variety of factors, many of which are beyond our control. Accordingly, you should not rely upon the results of any quarterly or annual periods as indications of future operating performance.
91
We do not intend to pay dividends on our common stock so any returns will be limited to the value of our stock.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Furthermore, future debt or other financing arrangements may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Any return to stockholders will therefore be limited to the appreciation of their stock.
We are controlled by our principal stockholders and management, which own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval, which will limit your ability to influence corporate matters.
Our executive officers, directors and 5% stockholders beneficially owned approximately [—]% of our voting stock as of the date of this Information Statement, and, upon the consummation of the Preferred Stock Conversion, that same group will continue to beneficially own approximately [—]% of our outstanding voting stock. Accordingly, these stockholders will have the ability to control us through this ownership position and significantly affect the outcome of all matters requiring stockholder approval. They effectively have the ability to determine all corporate actions requiring stockholder approval, including the election and removal of directors, any amendment to our Charter or Bylaws, or the approval of any merger or other significant corporate transaction, including a sale of substantially all of our assets. This could have the effect of delaying or preventing a change in control or otherwise discouraging a potential acquirer from attempting to obtain control of the Company, which could cause the market price of our common stock to decline or prevent stockholders from realizing a premium over the market price for common stock. Their interests may conflict with our interests as a company or the interests of our other stockholders.
We are an emerging growth company and a smaller reporting company and intend to take advantage of reduced disclosure requirements applicable to emerging growth companies, which could make the common stock less attractive to investors.
We are an “emerging growth company” (“EGC”) as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an EGC until the earliest to occur of (i) the last day of the fiscal year in which we have total annual gross revenue of $1.235 billion or more; (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of common stock pursuant to the registration statement; (iii) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period; or (iv) the date we qualify as a “large accelerated filer” under the rules of the SEC, which means the market value of the common stock held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter after we have been a reporting company in the United States for at least 12 months. For so long as we remain an EGC, we are permitted to and intend to rely upon exemptions from certain disclosure requirements that are applicable to other public companies that are not EGCs. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act (“SOX”).
We may take advantage of some, but not all, of the available exemptions available to EGCs. We cannot predict whether investors will find the common stock less attractive if we rely on these exemptions. If some investors find the common stock less attractive as a result, there may be a less active trading market for the common stock and the price of the common stock may be more volatile.
We are also a smaller reporting company, as defined in Rule 405 promulgated under the Securities Act (“SRC”). As an SRC, we intend to utilize certain reduced disclosure requirements, including publishing two years of audited financial statements instead of three years, as required for companies that do not qualify as an SRC. We will remain an SRC until the last day of the fiscal year in which we have (i) a public float that exceeded $250 million or (ii) annual revenues of more than $100 million and a public float that exceeded $700 million. To the extent we take advantage of such reduced disclosure obligations, it may make comparison of our financial statements to those of other public companies difficult or impossible.
After we cease to be an SRC, we are expected to incur additional management time and cost to comply with the more stringent reporting requirements applicable to companies that are accelerated filers or large accelerated filers, including complying with the auditor attestation requirements of Section 404 of SOX.
92
As a public company, we will be subject to more stringent federal law requirements.
As a public company, we will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of the NYSE American, and other applicable securities rules and regulations. Despite reforms made possible by the JOBS Act, compliance with these rules and regulations will nonetheless increase our legal and financial compliance costs, make some activities more difficult, time-consuming or costly and increase demand on our systems and resources, particularly after we are no longer an emerging growth company. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results.
As a result of disclosure of information in this Information Statement and in filings required of a public company, our business and financial condition will become more visible, which we believe may result in threatened or actual litigation, including by competitors and other third parties. If such claims are successful, our business, results of operations, financial condition and prospects could be harmed, and even if the claims do not result in litigation or are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and adversely affect our brand and reputation, business, results of operations, financial condition and prospects. We also expect that being a public company and the associated rules and regulations will make it more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain adequate coverage. These factors could also make it more difficult for us to attract and retain qualified members of our board of directors, particularly to serve on our audit committee and compensation committee, and qualified executive officers.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, and particularly after we are no longer an emerging growth company, we will incur significant legal, accounting, investor relations and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act and rules subsequently implemented by the SEC and the NYSE American have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Stockholder activism, the current political environment and the current high level of U.S. government intervention and regulatory reform may also lead to substantial new regulations and disclosure obligations, which may in turn lead to additional compliance costs and impact the manner in which we operate our business in ways we do not currently anticipate. Our management and other personnel will need to devote a substantial amount of time to comply with these requirements. Moreover, these requirements will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements.
We may not be able to satisfy listing requirements of the NYSE American or obtain or maintain a listing of our common stock on the NYSE American.
Our common stock has been approved for listing on the NYSE American, and after the consummation of this offering, we must meet certain financial and liquidity criteria to maintain such listing. If we violate the NYSE American listing requirements, our common stock may be delisted. If we fail to meet any of the NYSE American’s listing standards, our common stock may be delisted. In addition, our board of directors may determine that the cost of maintaining our listing on a national securities exchange outweighs the benefits of such listing. A delisting of our common stock from the NYSE American may materially impair our stockholders’ ability to buy and sell our common stock and could have an adverse effect on the market price of, and the efficiency of the trading market for, our common stock. The delisting of our common stock could significantly impair our ability to raise capital and the value of your investment.
93
If we fail to maintain proper and effective internal control over financial reporting, our ability to produce accurate and timely financial statements could be impaired, investors may lose confidence in our financial reporting and the trading price of our common stock may decline.
Pursuant to Section 404 of the Sarbanes-Oxley Act, we will be required to furnish a report by our management on our internal control over financial reporting, including an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. The rules governing the standards that must be met for management to assess our internal control over financial reporting are complex and require significant documentation, testing and possible remediation. To comply with the Sarbanes-Oxley Act, the requirements of being a reporting company under the Exchange Act and any complex accounting rules in the future, we may need to upgrade our information technology systems; implement additional financial and management controls, reporting systems and procedures; and hire additional accounting and finance staff. To date, we have had limited financial and accounting personnel to fully execute our accounting processes and address our internal control over financial reporting. If we are unable to hire the additional accounting and finance staff necessary to comply with these requirements, we may need to retain additional outside consultants. If we or, if required, our auditors, are unable to conclude that our internal control over financial reporting is effective, investors may lose confidence in our financial reporting and the trading price of our common stock may decline.
There can be no assurance that there will not be material weaknesses in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines that we have a material weakness in our internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline and we could be subject to sanctions or investigations by the NYSE American, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. For example, our directors or executive officers could inadvertently fail to disclose a new relationship or arrangement causing us to fail to make a required related party transaction disclosure. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
Our reported financial results may be adversely affected by changes in accounting principles generally accepted in the United States.
Generally accepted accounting principles in the United States are subject to interpretation by the Financial Accounting Standards Board, the SEC and various bodies formed to promulgate and interpret appropriate accounting principles. A change in these principles or interpretations could have a significant effect on our reported financial results, may retroactively affect previously reported results, could cause unexpected financial reporting fluctuations and may require us to make costly changes to our operational processes and accounting systems.
Claims for indemnification by our directors and officers may reduce our available funds to satisfy successful third-party claims against us and may reduce the amount of money available to us.
Our Charter and Bylaws provide that we will indemnify our directors and officers, in each case, to the fullest extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for any breach of fiduciary duties as directors, except liability for:
• any breach of the director’s duty of loyalty to the corporation or its stockholders;
• any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
94
• unlawful payments of dividends or unlawful stock repurchases or redemptions; or
• any transaction from which the director derived an improper personal benefit.
Such limitation of liability does not apply to liabilities arising under federal securities laws and does not affect the availability of equitable remedies such as injunctive relief or rescission.
Our Bylaws provide that we are required to indemnify our directors and officers to the fullest extent permitted by Delaware law and may indemnify our other employees and agents. Our Bylaws also provide that, on satisfaction of certain conditions, we will advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of Delaware law. We have entered and expect to continue to enter into agreements to indemnify our directors and executive officers. With certain exceptions, these agreements provide for indemnification for related expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by any of these individuals in connection with any action, proceeding or investigation. We believe that these Charter and Bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
While we maintain directors’ and officers’ liability insurance, such insurance may not be adequate to cover all liabilities that we may incur, which may reduce our available funds to satisfy third-party claims and may adversely impact our cash position.
Our failure to maintain effective internal controls over financial reporting could have an adverse impact on us.
We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition or results of operations. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting, disclosure of management’s assessment of our internal controls over financial reporting or disclosure of our public accounting firm’s attestation to or report on management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our shares.
In addition, discovery and disclosure of a material weakness in the future or our inability to cure the material weakness we previously discovered and disclosed, by definition, could have a material adverse impact on our financial statements. Such an occurrence could negatively affect our business and affect how our stock trades. This could, in turn, negatively affect our ability to access public equity or debt markets for capital.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on our company. If no securities or industry analysts commence coverage of our company, the trading price for our stock would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price may decline. If one or more of these analysts ceases coverage of our company or fails to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
95
Risks Related to the Merger and Related Transactions (Post-Closing)
The Merger may not produce the anticipated benefits, and the Company may be unable to successfully integrate the acquired business.
Although the Merger has been completed, the Company may not realize the anticipated benefits of the Merger, including expected synergies, growth opportunities, or cost savings. The Company’s ability to achieve these benefits depends on a number of factors, including the successful integration of the MindWave business, which may be more difficult, time-consuming or costly than expected.
The Company may incur additional costs and liabilities arising from the Merger.
Following the closing of the Merger, the Company may continue to incur significant costs related to integration, restructuring, professional fees, and other transaction-related expenses. In addition, the Company may be subject to liabilities arising from the MindWave business that were not known or fully quantified at the time the Merger Agreement was entered into.
The Merger Agreement may continue to affect the Company’s operations and capital structure.
Certain provisions of the Merger Agreement, including those governing the issuance and the conversion of the Preferred Stock, continue to apply following the closing of the Merger and may limit the Company’s flexibility with respect to capital structure, financings, or other corporate actions.
Risks Related to the Reverse Stock Split
The reverse stock split may not result in a sustained increase in the market price of the Common Stock.
There can be no assurance that the reverse stock split will result in an increase in the market price of the Common Stock for any meaningful period. If the market price does not increase proportionally, the Company’s market capitalization could decline.
The reverse stock split may reduce the liquidity of the Common Stock.
The reverse stock split will reduce the number of outstanding shares of Common Stock, which could reduce liquidity and increase volatility in the trading price of the Common Stock.
Stockholders will not experience an increase in proportional ownership.
Although the reverse stock split will proportionally reduce the number of shares of Common Stock held by each stockholder, it will not change the stockholder’s ownership percentage in the Company.
Risks Related to Equity Incentive Plans
Equity incentive plans may result in additional dilution.
The adoption of a new equity incentive plan or amendments to an existing equity incentive plan may result in the issuance of additional shares of Common Stock or equity awards, which would further dilute existing stockholders.
Equity-based compensation may increase expenses without corresponding benefits.
Equity-based compensation may increase the Company’s compensation expenses and may not result in improved performance, retention, or stockholder value.
96
Risks Related to MindWave’s Business
MindWave’s quarterly operating results, revenues, and expenses may fluctuate significantly, which could have an adverse effect on the market price of our listed securities.
MindWave’s quarterly operating results, revenues, and expenses are subject to significant variability, and its historical financial results may not be indicative of future performance. These fluctuations could adversely affect the market price of our listed securities.
MindWave’s results of operations may fluctuate significantly from quarter to quarter due to a variety of factors, including:
• fluctuations in the price of bitcoin and the associated impact of fair value accounting under ASU 2023-08;
• changes in demand for bitcoin-backed lending products, including borrower defaults, prepayments, or collateral liquidations;
• recognition of gains or losses upon the sale of bitcoin, if we choose to sell bitcoin to meet liquidity needs or other strategic objectives;
• regulatory, commercial, and technical developments related to bitcoin, the Bitcoin blockchain, or the digital asset lending industry more broadly;
• the incurrence of fixed obligations, including interest expense on debt, dividend obligations on any preferred stock we may issue, office leases, and personnel costs;
• the impact of macroeconomic and global events — including inflation, rising interest rates, war, terrorism, infectious diseases (such as COVID-19), natural disasters, and government responses to such events — on the global economy and the market for and price of bitcoin;
• adjustments to our tax liabilities, deferred tax balances, or valuation allowances, which may vary based on profitability and fair value changes in bitcoin; and
• increases or decreases in our unrecognized tax benefits.
Because of these factors, quarter-to-quarter comparisons of MindWave’s operating results may not be a reliable indicator of future performance. It is possible that in one or more future quarters, MindWave’s operating results may fall below the expectations of public market analysts or investors. If this occurs, the market price of our listed securities could decline.
Limited ability to adjust expense and reliance on Bitcoin sales to meet liquidity needs.
MindWave’s operating expense budgets are based on expected revenue trends and strategic objectives. Many of its expenses, such as interest expense on borrowings, personnel costs, office leases, and regulatory compliance obligations, are relatively fixed in the short term. As a result, we may be unable to reduce spending quickly enough to offset an unexpected shortfall in revenues, which could adversely affect our financial condition and results of operations. To meet liquidity needs under such circumstances, we may be required to sell bitcoin, raise additional debt or equity financing, or take other actions. Reliance on bitcoin sales exposes us to the volatility of the bitcoin market, and periods of sharp declines in bitcoin prices could force us to sell at unfavorable times or values, potentially resulting in realized losses and reduced capital resources. In addition, the relative illiquidity of the bitcoin market compared to traditional capital markets could make it difficult to liquidate large positions without negatively impacting the market price of bitcoin. Any such sales or financings could cause significant variation in our quarterly operating results and, in the case of equity financing, may be dilutive to existing stockholders.
97
MindWave’s reliance on Bitcoin sales to meet liquidity needs may expose us to volatility and adverse market conditions.
MindWave holds the majority of its assets in bitcoin. To the extent we are required to liquidate bitcoin holdings to fund operations, meet liquidity needs, or service debt obligations, we will be subject to the volatility of the bitcoin market. Periods of sharp declines in bitcoin prices could force us to sell at unfavorable times or values, potentially resulting in realized losses and reduced capital resources. In addition, the size and relative illiquidity of the bitcoin market compared to traditional capital markets could make it difficult for us to liquidate large positions without negatively impacting the market price of bitcoin. Any such forced sales could materially and adversely affect our financial condition and operating results.
MindWave may not be able to achieve or maintain profitability in future periods.
MindWave has not yet achieved profitability, and there can be no assurance that it will become profitable in future periods. MindWave’s ability to achieve and maintain profitability depends on a number of factors, many of which are outside its control, including fluctuations in the price of bitcoin, demand for its bitcoin-backed products, borrower defaults, regulatory developments, and the volatility of its reported earnings under recently adopted accounting guidance.
In December 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2023-08, Accounting for and Disclosure of Crypto Assets. This guidance requires entities to measure crypto assets, including bitcoin, at fair value with changes recognized in net income each reporting period. As a result, MindWave’s financial results may be subject to significant volatility, and may report material losses in periods of bitcoin price declines. Conversely, periods of bitcoin price appreciation may result in reported gains. This volatility could make it more difficult for MindWave to achieve consistent profitability from period to period.
As of [—], MindWave did not have any deferred tax assets. If, in future periods, MindWave generates deferred tax assets and the market value of bitcoin at a reporting date is less than the average cost basis of its bitcoin holdings, MindWave may be required to establish a valuation allowance against such deferred tax assets. Any significant increase in a valuation allowance could result in a charge that would materially adversely affect MindWave’s net income for the period in which the charge is recorded.
If MindWave is unable to achieve or maintain profitability, its results of operations and financial condition could be materially adversely affected, and the market price of our securities could decline.
MindWave’s bitcoin strategy exposes us to risks associated with bitcoin, including changes in accounting treatment of our holdings that could increase the volatility of our results.
MindWave’s strategy to hold bitcoin as a treasury asset exposes us to a number of risks, including the following:
Bitcoin is a highly volatile asset. Bitcoin has experienced extreme price volatility. The trading price of bitcoin has significantly decreased in prior periods, and similar declines may occur again in the future, which could adversely affect our financial results and the market price of our securities.
Bitcoin does not generate cash flows. Bitcoin does not pay interest or dividends. MindWave can only generate liquidity from its bitcoin holdings if they sell bitcoin or use it as collateral to support income-generating strategies. Even if they pursue such strategies, there is no assurance that they will generate sufficient cash flow or that they will not expose them to additional risks, including borrower defaults, counterparty risk, or regulatory restrictions.
MindWave’s bitcoin holdings significantly impact our financial results and the market price of our listed securities. MindWave’s bitcoin holdings materially affect our financial results. If MindWave continues to increase its overall bitcoin holdings, the impact of price fluctuations in bitcoin on our results of operations and on the market price of our securities will likely become even greater.
MindWave’s assets are concentrated in bitcoin. The majority of MindWave’s assets are concentrated in bitcoin. This lack of diversification exposes us to greater risk than companies whose treasury assets consist of a more traditional or diversified portfolio.
98
MindWave relies on access to equity and debt financing to support their bitcoin strategy. We expect to finance, purchases of bitcoin and business operations primarily through equity and debt financings. Our ability to raise financing on favorable terms depends in part on the value of our bitcoin holdings, market sentiment toward bitcoin, and investor perception of our business. If we cannot obtain additional financing on favorable terms, or at all, we may not be able to successfully execute on our bitcoin strategy.
MindWave’s bitcoin strategy is unproven over the long term. MindWave’s strategy of holding bitcoin as a treasury reserve has not been tested over an extended period of time or under a wide range of market conditions. While we believe bitcoin has long-term potential as a store of value and hedge against inflation, in the short term bitcoin’s price has declined during periods of rising inflation. If MindWave’s bitcoin strategy proves unsuccessful, our financial condition, results of operations, and the market price of our securities could be materially adversely affected.
The broader digital assets industry is subject to counterparty and systemic risks. Recent bankruptcies, liquidations, and regulatory enforcement actions in the digital assets industry have negatively impacted confidence in digital asset markets and, in the short term, the adoption and use of bitcoin. Future failures of participants in the digital asset industry could further negatively impact the adoption, price, and use of bitcoin, limit our ability to access financing collateralized by bitcoin, or increase counterparty risks applicable to our business.
The digital assets industry is rapidly evolving. The legal, regulatory, technical, and accounting environment for digital assets continues to evolve. Changes in adoption rates, technology, regulation, accounting treatment, or market perception could materially and adversely impact bitcoin, MindWave’s business, and our business in ways that cannot currently be predicted.
Bitcoin is a highly volatile asset, and fluctuations in its price have in the past and are likely in the future to materially influence our financial results and the market price of our Common Stock.
Bitcoin is a highly volatile asset. MindWave’s financial results and the market price of our listed securities would be adversely affected, and our business and financial condition would be negatively impacted, if the price of bitcoin were to decrease substantially (as it has in the past, including during 2022). Factors that may cause the price of bitcoin to decrease include, but are not limited to:
• Market activity and trading dynamics. Volatility driven by retail and institutional speculators, actual or expected sales by large holders (including court-ordered liquidations following bankruptcies, hacks, or seizures), and manipulation of spot or derivative bitcoin markets or spot bitcoin exchange-traded products (“ETPs”).
• Negative publicity and sentiment. Adverse media coverage or public perception that bitcoin is used for illicit purposes (such as sanctions evasion, criminal financing, or terrorism), regulatory actions against leading market participants (such as Coinbase or Binance), the bankruptcies of digital asset companies (such as FTX, BlockFi, or Celsius), or heightened concerns over bitcoin’s environmental impact.
• Competition and substitution. The emergence of alternative digital assets with greater speed, scalability, or energy efficiency, including stablecoins, central bank digital currencies, or asset-backed tokens, that divert adoption away from bitcoin.
• Systemic industry failures. Insolvency of major custodians, exchanges, lenders, or banks serving the digital assets sector (e.g., Silvergate, Signature, Prime Trust), or exits of significant market participants, which reduce confidence and liquidity in digital asset markets.
• Technological and protocol risks. Changes or failures in the Bitcoin protocol, such as consensus bugs, scalability issues, or contested upgrades; reductions in mining rewards due to halving events (most recently in April 2024); rising mining costs; or advances in mathematics or computing (such as quantum computing) that could undermine the cryptography securing the Bitcoin blockchain.
• Regulatory, legislative, and enforcement actions. Restrictions on ownership, transfer, custody, trading, or use of bitcoin; enforcement actions against exchanges or service providers; or changes in tax treatment that reduce demand or liquidity.
99
• Macroeconomic and geopolitical conditions. Rising interest rates, inflationary or deflationary pressures, fiscal and monetary policy shifts, trade restrictions, fiat currency devaluations, sanctions regimes, wars, terrorism, pandemics, and political instability (including the ongoing Russia-Ukraine conflict and instability in the Middle East).
• Operational challenges. Congestion on the Bitcoin network, increased transaction fees, or loss of confidence in bitcoin’s functionality as a medium of exchange or store of value.
Because bitcoin price volatility directly affects the fair value of MindWave’s treasury holdings, substantial decreases in bitcoin’s price could have a disproportionate impact on MindWave’s financial condition, results of operations, and the market price of our securities.
Bitcoin and other digital assets are novel assets, and are subject to significant legal, commercial, regulatory, and technical uncertainty.
Bitcoin and other digital assets are relatively novel and remain subject to significant uncertainty, which could adversely affect their price and adoption. Because MindWave’s business strategy includes holding bitcoin and providing bitcoin-backed lending, developments affecting the legal, commercial, regulatory, or technical status of bitcoin or other digital assets could materially impact MindWave’s financial condition, results of operations, and the market price of our securities.
Legal and regulatory uncertainty. The application of state, federal, and foreign securities laws and other laws to digital assets is unclear in certain respects, and regulators may interpret or apply existing laws in ways that adversely affect the price of bitcoin or MindWave’s ability to own, hold, or transfer bitcoin. The U.S. federal government, state governments, regulatory agencies, and foreign jurisdictions may enact new laws or pursue enforcement, legislative, or judicial actions that restrict digital asset activities. Recent examples include:
• the SEC’s enforcement actions against Coinbase, Inc., Binance Holdings Ltd., and Kraken, alleging violations of securities laws;
• the settlement in November 2023 requiring Binance to pay $4.3 billion in penalties and to exit the U.S. market;
• the European Union’s adoption of the Markets in Crypto Assets Regulation (“MiCA”), a comprehensive framework for digital asset issuance and use;
• the United Kingdom’s adoption of the Financial Services and Markets Act 2023 (“FSMA 2023”), extending regulation to “cryptoassets”; and
• the People’s Bank of China declaring all cryptocurrency transactions illegal and banning mining.
It is not possible to predict whether or when new laws will be enacted, how existing laws will be applied, or how additional regulatory oversight may impact digital asset markets, service providers, or MindWave’s own operations. Such developments could reduce liquidity, discourage institutional or retail participation in digital assets, or negatively affect MindWave’s ability to hold or transact in bitcoin.
Technical uncertainty. Bitcoin has no physical existence beyond the record of transactions on its blockchain. A number of technical factors could undermine confidence in bitcoin and reduce its price, including:
• malicious attacks by miners or other bad actors;
• inadequate mining rewards or fees that discourage transaction validation;
• hard “forks” creating competing blockchains;
• advances in mathematics or computing, including quantum computing, that undermine the cryptography securing the Bitcoin network; and
• congestion, high transaction fees, or loss of confidence in the usability of bitcoin.
100
Financial services uncertainty. The liquidity of bitcoin and the broader digital asset market may be reduced if banks and financial institutions deny or limit services to businesses that hold or transact in digital assets. Past regulatory actions, such as the February 2023 “Interagency Liquidity Risk Statement” issued by U.S. banking regulators, have contributed to reduced access to banking services for digital asset businesses. Similar restrictions in the future could limit access to financial services for bitcoin-related businesses, further reducing liquidity and confidence in the market.
Because MindWave’s business model depends on bitcoin as a treasury asset, any legal, commercial, regulatory, or technical developments that negatively impact the price or adoption of bitcoin could materially and adversely affect MindWave’s financial condition, results of operations, and the market price of our securities.
MindWave’s limited operating history and adoption of new accounting guidance may cause significant variability in reported earnings relating to MindWave’s bitcoin holdings.
MindWave’s limited operating history means its historical financial statements do not reflect the potential variability in earnings that may result from its bitcoin strategy. The price of bitcoin has historically been highly volatile, experiencing dramatic price fluctuations within short periods of time, and this volatility is likely to materially impact our financial results in future periods.
In December 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2023-08, requires entities holding crypto assets, such as bitcoin, to measure such assets at fair value at the end of each reporting period and to recognize changes in fair value through net income.
Further, MindWave intends to purchase additional bitcoin over time. As the proportion of its assets represented by bitcoin increases, the impact of bitcoin price movements on reported financial results will also increase. Accordingly, our future earnings may fluctuate significantly from period to period, and investors should not rely on MindWave’s historical financial results as an indication of future performance.
The availability of spot ETPs for bitcoin and other digital assets may adversely affect the market price of our listed securities.
Although bitcoin and other digital assets have experienced a surge of investor attention since bitcoin was introduced in 2008, until recently U.S. investors had limited means to gain direct exposure to bitcoin through traditional investment channels. Most investors either held bitcoin directly through hosted or unhosted wallets or sought exposure through private placement investment vehicles. Historically, such private vehicles often traded at a premium to net asset value, in part because of the scarcity of traditional investment products providing exposure to bitcoin.
On January 10, 2024, the SEC approved the listing and trading of spot bitcoin exchange-traded products (“ETPs”), which commenced trading on January 11, 2024, with significant volume on the first day. On May 23, 2024, the SEC also approved rule changes permitting the listing and trading of spot ETPs for ether, which began trading in July 2024. These approvals have provided investors with accessible, low-cost options to gain direct exposure to bitcoin and other digital assets through traditional brokerage accounts.
To the extent that investors perceive our securities as a substitute for spot bitcoin ETPs, demand for our stock may decline if investors instead choose to allocate capital to such ETPs, which may be viewed as offering “pure play” exposure to bitcoin with certain structural advantages. For example, unlike spot bitcoin ETPs:
• our shares of Common Stock are subject to federal income taxation at the corporate level;
• we do not seek to track the value of bitcoin or operate under a trust structure that limits us to a single stated investment objective; and
• our shares of Common Stock are not continuously aligned to bitcoin’s market price through the creation and redemption mechanisms available to ETPs.
Investor comparisons to ETPs and other investment vehicles offering exposure to bitcoin may affect how our stock is valued in the market. As a result, the availability of spot ETPs for bitcoin and other digital assets could adversely affect the trading price of our shares of Common Stock.
101
MindWave’s bitcoin strategy subjects us to enhanced regulatory oversight.
Several spot bitcoin exchange-traded products (“ETPs”) have recently received approval from the SEC to list their shares on U.S. national securities exchanges with continuous share creation and redemption at net asset value. Although MindWave is not, and does not function in the manner of, a spot bitcoin ETP, its strategy of acquiring and holding bitcoin may nevertheless attract regulatory scrutiny from the SEC or other federal and state agencies.
There has also been increasing regulatory and public focus on the extent to which digital assets may be used to launder proceeds of illegal activities, finance criminal or terrorist organizations, or evade sanctions regimes, including those adopted in response to the ongoing conflict between Russia and Ukraine. While MindWave maintains policies and procedures reasonably designed to promote compliance with applicable anti-money laundering and sanctions laws and plan to acquire bitcoin only through U.S.-regulated entities subject to AML requirements, if it were determined that it purchased bitcoin from a bad actor or sanctioned person, it could be subject to significant regulatory proceedings and restrictions or prohibitions on further transactions.
MindWave intends to acquire bitcoin in the future. Should it incur indebtedness or enter into financial arrangements collateralized by its bitcoin holdings, or pursue strategies designed to generate income streams or liquidity from its bitcoin, such transactions could subject us to heightened regulatory oversight, including potential compliance obligations under federal and state money services business regulations, money transmitter licensing requirements, and securities or commodities laws.
Following the collapse and Chapter 11 bankruptcy filing of FTX in November 2022, regulators in the U.S. and abroad have increased their scrutiny of the digital assets industry. While the FTX collapse did not directly impact our business, it has contributed to heightened enforcement activity and calls for new or revised regulatory frameworks. Future regulatory actions, changing interpretations of existing laws, or new legislation could significantly limit our ability to hold or transact in bitcoin or impose additional compliance costs.
In addition, private market participants wary of bitcoin-related risks have restricted access to securities with bitcoin exposure. For example, an affiliate of HSBC Holdings prohibited customers of its HSBC InvestDirect retail investment platform from purchasing securities of an issuer with significant bitcoin exposure. Similar actions by other financial institutions, broker-dealers, or custodians could adversely affect the liquidity of our securities or the market price of our stock.
Due to the unregulated nature and lack of transparency surrounding the operations of many bitcoin trading venues, such venues may experience greater fraud, security failures, or regulatory or operational problems than trading venues for more established asset classes, which may result in a loss of confidence in bitcoin trading venues and adversely affect the value of MindWave’s future bitcoin holdings.
Bitcoin trading venues are relatively new and, in many cases, unregulated. Many do not provide the public with meaningful transparency regarding their ownership structure, management teams, corporate practices, or regulatory compliance. As a result, the marketplace may lose confidence in bitcoin trading venues, including even prominent exchanges that handle significant volumes of bitcoin trading and are subject to regulatory oversight. If one or more major trading venues were to cease or pause operations for a prolonged period, or experience fraud, security breaches, large withdrawal volumes, or other operational problems, the price of bitcoin could decline substantially.
For example, reports published in 2019 suggested that as much as 80 – 95% of reported bitcoin trading volume was false or non-economic in nature, primarily on unregulated offshore exchanges. More recently, the SEC’s June 5, 2023 complaint against Binance Holdings Ltd. alleged that Binance engaged in “wash trading” through affiliates to artificially inflate reported trading volumes. The SEC has also brought actions against individuals and digital asset platforms alleging similar manipulative practices. These allegations and findings suggest that the true size and depth of the bitcoin market may be significantly smaller than commonly believed. Any actual or perceived fraudulent or manipulative activity in the bitcoin markets could adversely impact the price of bitcoin and, correspondingly, the value of any bitcoin we acquire in the future.
102
Moreover, the collapse or disruption of other key participants in the bitcoin ecosystem — including trading venues, lending platforms, custodians, or institutional investors — could materially reduce confidence in bitcoin. For instance, in 2022, Celsius Network, Voyager Digital, Three Arrows Capital, FTX, and BlockFi each filed for bankruptcy, events that contributed to sharp declines in the market prices of bitcoin and other digital assets. Similarly, in 2023, the SEC brought enforcement actions against Coinbase, Binance, and Kraken, which were followed by significant price declines in bitcoin and other digital assets. If similar failures or regulatory actions occur in the future, they could negatively affect bitcoin markets and, as a result, the value of MindWave’s bitcoin holdings and our stock price.
The concentration of MindWave’s bitcoin holdings will enhance the risks inherent in our bitcoin strategy.
Concentrating a substantial portion of our treasury assets in bitcoin exposes us to the full volatility of a single, highly speculative asset class and limits the diversification that could otherwise mitigate risk. The price of bitcoin has historically experienced significant fluctuations, including in 2022 when it lost more than half its market value. Any similar or future decline in the price of bitcoin would have a proportionally greater adverse effect on our financial condition, liquidity, and results of operations than if we held a diversified portfolio of assets.
As MindWave’s bitcoin holdings grow, the volatility and risks associated with bitcoin are expected to become increasingly pronounced in our financial statements and may contribute to volatility in the market price of our common stock.
The emergence or growth of other digital assets, including those with significant private or public sector backing, could have a negative impact on the price of bitcoin and adversely affect MindWave’s business.
MindWave intends to pursue a bitcoin acquisition and treasury strategy, which will result in a concentration of its assets in bitcoin. Accordingly, the emergence or growth of other digital assets could have a material adverse effect on MindWave’s future financial condition and results of operations.
As of July 2025, bitcoin remains the largest digital asset by market capitalization. However, there are numerous alternative digital assets, and many entities — including financial institutions and consortiums — are investing in private or permissioned blockchain platforms or alternative validation mechanisms. For example, in 2022 the Ethereum network transitioned from proof-of-work mining to a “proof-of-stake” mechanism, which requires significantly less computing power and has since undergone additional upgrades. If proof-of-stake or other mechanisms are perceived as more efficient, sustainable, or scalable than bitcoin’s proof-of-work system, alternative digital assets may gain market share relative to bitcoin.
Other digital assets, such as “stablecoins,” are designed to maintain a constant value, often pegged to the U.S. dollar and backed by reserves of liquid assets like U.S. Treasury securities. Stablecoins have grown rapidly as a medium of exchange and store of value on digital asset trading platforms. As of 2024, two of the eight largest digital assets by market capitalization were U.S. dollar-pegged stablecoins.
In addition, central banks in various jurisdictions are exploring or introducing central bank digital currencies (“CBDCs”). For example, China launched its CBDC pilot to consumers in 2022, and governments including the United States, the United Kingdom, the European Union, and Israel continue to evaluate the development of their own CBDCs. CBDCs, as legal tender in their respective jurisdictions, could compete with or even displace bitcoin as a medium of exchange or store of value.
As a result, the emergence or growth of other digital assets, including stablecoins and CBDCs, could reduce demand for bitcoin and adversely affect its market price, which in turn could have a material adverse impact on MindWave’s business, strategy, and financial condition.
103
A cyberattack or significant disruption of MindWave’s information technology systems could adversely affect its business, results of operations, and financial condition
MindWave relies heavily on information technology systems for administrative and general operational management. A cyberattack, data breach, prolonged outage, or other significant disruption of MindWave’s IT systems, or those of its vendors and partners, could impair its ability to process transactions, service customers, or safeguard collateral.
Because MindWave also collects, transmits, and sensitive information, any unauthorized access, exfiltration, or alteration of such information could result in reputational harm, litigation, regulatory investigations, and significant remediation costs. Outsourcing portions of MindWave’s IT infrastructure to third-party providers increases MindWave’s exposure to risks originating in its vendors’ systems, including potential supply-chain attacks.
While MindWave will maintain cybersecurity protocols and carry cyber liability insurance, the sophistication and frequency of attacks — often by state actors, organized criminal groups, and other well-funded actors — makes it impossible to guarantee prevention of all intrusions. Insurance coverage may be insufficient to compensate us for all losses. Any such attack or disruption could materially and adversely affect MindWave’s operations, financial condition, and ability to grow.
Cyberattacks directed at digital asset companies have become more frequent and sophisticated, with some attacks linked to state-sponsored groups. Ongoing geopolitical conflicts may heighten the risk of systemic cyber incidents, including malware or ransomware targeting custodians or exchanges. If MindWave or its partners are subject to such attacks it could have material adverse effects on its operations.
Changes in laws or regulations relating to privacy or the collection, processing, disclosure, storage, localization, or transmission of personal data, or any actual or perceived failure by us or our third-party service providers to comply with such laws and regulations, contractual obligations, or applicable privacy policies, could materially adversely affect our business
Certain aspects of our business involve collecting, processing, disclosing, storing, and transmitting personal data, which are subject to certain privacy policies, contractual obligations, and U.S. and foreign laws, regulations, and directives relating to privacy and data protection. In addition, the types of data subject to protection as personal data in the European Union, China, the United States, and elsewhere have been expanding. In recent years, the collection and use of personal data by companies have come under increased regulatory and public scrutiny, especially in relation to the collection and processing of sensitive data, such as healthcare, biometric, genetic, financial services, and children’s data, precise location data, and data regarding a person’s race or ethnic origins, political opinions, or religious beliefs.
There are various enforcement agencies at both the state and federal level that review compliance with these requirements, including the United States Department of Health and Human Services for potential violations of the Health Insurance Portability and Accountability Act of 1996 and the Federal Trade Commission (“FTC”). If we are subject to a potential FTC enforcement action, we may be subject to a settlement order that requires us to adhere to very specific privacy and data security practices, which may impact our business. We may also be required to pay fines as part of a settlement (depending on the nature of the alleged violations). If we violate any consent order that we reach with the FTC, we may be subject to additional fines and compliance requirements. We face risks of similar enforcement from State Attorneys General and, potentially, other regulatory agencies.
Similar laws exist in other foreign jurisdictions, including the European Union, that may impact our business activities. In addition, various U.S. federal and state government agencies and foreign government bodies may enact new or additional laws or regulations, or issue rulings that invalidate prior laws or regulations, concerning privacy, data storage, data protection, and cross-border transfer of data that could materially adversely impact our business.
Any systems failure or security breach that results in the release of, or unauthorized access to, personal data, or any failure or perceived failure by us or our third-party service providers to comply with applicable privacy policies, contractual obligations, or any applicable laws or regulations relating to privacy or data protection, could result in proceedings against us by domestic or foreign government entities or others, including private plaintiffs in litigation. Such proceedings could result in the imposition of sanctions, fines, penalties, liabilities, government orders, and/or orders requiring that we change our data practices, any of which could have a material adverse effect on our business, operating results, reputation, and financial condition.
104
Furthermore, the U.S. Congress is considering comprehensive privacy legislation. At this time, it is unclear whether Congress will pass such a law and if so, when and what it will require and prohibit. Moreover, it is not clear whether any such legislation would give the FTC any new authority to impose civil penalties for violations of the Federal Trade Commission Act in the first instance, whether Congress will grant the FTC rulemaking authority over privacy and information security, or whether Congress will vest some or all privacy and data security regulatory authority and enforcement power in a new agency, akin to EU data protection authorities.
Complying with these and other changing requirements could cause us or our customers to incur substantial costs or pay substantial fines or penalties, require us to change our business practices, require us to take on more onerous obligations in our contracts, or limit our ability to provide certain offerings in certain jurisdictions, any of which could materially adversely affect our business and operating results. New laws or regulations restricting or limiting the collection or use of mobile data could also reduce demand for certain of our offerings or require changes to our business practices, which could materially adversely affect our business and operating results.
105
unaudited pro forma financial information
The following unaudited pro forma combined financial information presents the unaudited pro forma combined balance sheet and statement of operations based upon the combined historical financial statements of the Company and MindWave after giving effect to the Mergers and the adjustments described in the accompanying notes.
The unaudited pro forma combined balance sheets of the Company and MindWave as of September 30, 2025 have been prepared to reflect the effects of the Mergers as if they occurred on September 30, 2025. The unaudited pro forma combined statements of operations for the Company and MindWave for the six months ended September 30, 2025 combine the historical results and operations of the Company and APUS giving effect to the Mergers as if they occurred on April 1, 2025. The unaudited pro forma combined statements of operations for the Company and MindWave for the twelve months ended March 31, 2025 combine the historical results and operations of the Company and APUS giving effect to the Mergers as if they occurred on April 1, 2024.
The fiscal year end of MindWave is March 31, 2025, which was used as the basis of presentation in the accompanying pro forma financial statements.
The unaudited pro forma combined financial information should be read in conjunction with the audited and unaudited historical financial statements of the Company and APUS and the notes thereto. Additional information about the basis of presentation of this information is provided in Note 2 below.
The unaudited pro forma combined financial information has been prepared in accordance with Article 11 of Regulation S-X. Although the Company is the legal acquirer, the Merger is accounted for as a reverse acquisition under US GAAP, with MindWave Innovations Inc. identified as the accounting acquirer due to its dominant controlling interest. For accounting purposes, MindWave is treated as the acquiring entity, and the Company’s identifiable assets and liabilities are measured at their estimated fair values as of the closing date.
The preliminary purchase consideration is measured using the deemed issuance method, representing the fair value of the equity interests retained by the original stockholders of APUS. This consideration has been allocated on a provisional basis, resulting in the recognition of goodwill for the excess of the purchase price over the fair value of the identifiable net assets. These amounts are subject to further adjustment during the measurement period as the Company finalizes its valuations and completes the accounting for the business combination.
The unaudited pro forma combined financial information is provided for informational purposes only and is not necessarily indicative of the operating results or financial position that would have occurred if the transaction had been completed as of the dates set forth above, nor is it indicative of the future results or financial position of the combined company. In connection with the pro forma financial information, the Company allocated the purchase price using its best estimates of fair value. Accordingly, the pro forma acquisition price adjustments are preliminary and subject to further adjustments as additional information becomes available and as additional analyses are performed. The unaudited pro forma combined financial information also does not give effect to the potential impact of current financial conditions, any anticipated synergies, operating efficiencies or cost savings that may result from the transaction or any integration costs. Furthermore, the unaudited pro forma combined statements of operations do not include certain nonrecurring charges and the related tax effects which result directly from the transaction as described in the notes to the unaudited pro forma combined financial information.
106
Unaudited Proforma Combined Statement of Operations for the Six Months Ended September 30, 2025
|
MindWave |
APUS |
Pro Forma |
Pro Forma |
|||||||||||||||
|
Revenue |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
||||||
|
|
|
|
|
|
|
|
|
|||||||||||
|
Operating expense (income): |
|
|
|
|
|
|
|
|
||||||||||
|
General and administrative |
|
3,371,133 |
|
|
3,236,666 |
|
|
— |
|
|
6,607,799 |
|
||||||
|
Research and development expenses |
|
|
|
1,271,477 |
|
|
— |
|
|
1,271,477 |
|
|||||||
|
Distribution and commission expenses |
|
4,634,172 |
|
|
— |
|
|
— |
|
|
4,634,172 |
|
||||||
|
Trading gains, net |
|
(3,300 |
) |
|
— |
|
|
— |
|
|
(3,300 |
) |
||||||
|
Realized gain on sale of digital assets |
|
(10,479,790 |
) |
|
— |
|
|
— |
|
|
(10,479,790 |
) |
||||||
|
Unrealized gain from changes in fair value of digital assets |
|
9,066,549 |
|
|
— |
|
|
— |
|
|
9,066,549 |
|
||||||
|
Total operating expense (income) |
|
6,588,764 |
|
|
4,508,143 |
|
|
— |
|
|
11,096,907 |
|
||||||
|
|
|
|
|
|
|
|
|
|||||||||||
|
Loss from operations |
|
(6,588,764 |
) |
|
(4,508,143 |
) |
|
— |
|
|
(11,096,907 |
) |
||||||
|
|
|
|
|
|
|
|
|
|||||||||||
|
Other income (expense) |
|
|
|
|
|
|
|
|
||||||||||
|
Change in fair value of warrant liability |
|
— |
|
|
22,377 |
|
|
— |
|
|
22,377 |
|
||||||
|
Interest income |
|
— |
|
|
71,673 |
|
|
— |
|
|
71,673 |
|
||||||
|
Interest expense |
|
— |
|
|
(29,355 |
) |
|
(261,000 |
) |
(D) |
|
(290,355 |
) |
|||||
|
Total other income (expense) |
|
— |
|
|
64,695 |
|
|
(261,000 |
) |
|
(196,305 |
) |
||||||
|
Net loss |
$ |
(6,588,764 |
) |
$ |
(4,443,448 |
) |
$ |
(261,000 |
) |
$ |
(11,293,212 |
) |
||||||
|
|
|
|
|
|
|
|
|
|||||||||||
|
Net loss per share – basic and diluted |
|
|
|
|
|
|
$ |
(0.07 |
) |
|||||||||
|
Weighted average common shares outstanding – basic and diluted |
|
|
|
|
|
|
|
162,116,314 |
|
|||||||||
107
Unaudited Proforma Combined Statement of Operations for the Year Ended March 31, 2025
|
MindWave |
APUS |
Pro Forma |
Pro Forma |
|||||||||||||||
|
Revenue |
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
||||||
|
|
|
|
|
|
|
|
|
|||||||||||
|
Operating (income) expense: |
|
|
|
|
|
|
|
|
||||||||||
|
General and administrative |
|
2,674,157 |
|
|
1,367,737 |
|
|
— |
|
|
4,041,894 |
|
||||||
|
Distribution and commission expenses |
|
3,718,158 |
|
|
— |
|
|
— |
|
|
3,718,158 |
|
||||||
|
Realized gain on sale of digital assets |
|
(8,360,488 |
) |
|
— |
|
|
— |
|
|
(8,360,488 |
) |
||||||
|
Unrealized gain from changes in fair value of digital assets |
|
(53,930,184 |
) |
|
— |
|
|
— |
|
|
(53,930,184 |
) |
||||||
|
Total operating (income) expense |
|
(55,898,357 |
) |
|
1,367,737 |
|
|
— |
|
|
(54,530,620 |
) |
||||||
|
|
|
|
|
|
|
|
|
|||||||||||
|
Income (loss) from operations |
|
55,898,357 |
|
|
(1,367,737 |
) |
|
— |
|
|
54,530,620 |
|
||||||
|
|
|
|
|
|
|
|
|
|||||||||||
|
Other income (expense) |
|
|
|
|
|
|
|
|
||||||||||
|
Interest income |
|
— |
|
|
666 |
|
|
— |
|
|
666 |
|
||||||
|
Interest expense |
|
— |
|
|
(128,843 |
) |
|
(522,000 |
) |
|
(650,843 |
) |
||||||
|
Total other income (expense) |
|
— |
|
|
(128,177 |
) |
|
(522,000 |
) |
|
(650,177 |
) |
||||||
|
Net (loss) income |
$ |
55,898,357 |
|
$ |
(1,495,914 |
) |
$ |
(522,000 |
) |
$ |
53,880,443 |
|
||||||
|
Net (loss) income per share – basic and diluted |
|
|
|
|
|
|
$ |
0.34 |
|
|||||||||
|
Weighted average common shares outstanding – basic and diluted |
|
|
|
|
|
|
|
157,444,181 |
|
|||||||||
108
Unaudited Proforma Combined Balance Sheet as of September 30, 2025
|
MindWave |
APUS |
Pro Forma |
Pro Forma |
|||||||||||||
|
ASSETS |
|
|
|
|
|
|
||||||||||
|
Current assets: |
|
|
|
|
|
|
||||||||||
|
Cash and cash equivalents |
$ |
6 |
$ |
6,986,617 |
|
$ |
12,006,000 |
|
(C) |
$ |
18,992,623 |
|||||
|
Prepaid expenses and other current assets |
|
— |
|
2,099,491 |
|
|
— |
|
|
2,099,491 |
||||||
|
Digital assets, at fair value |
|
3,300 |
|
— |
|
|
— |
|
|
3,300 |
||||||
|
Total current assets |
|
3,306 |
|
9,086,108 |
|
|
12,006,000 |
|
|
21,095,414 |
||||||
|
Digital assets, at fair value |
|
132,273,773 |
|
— |
|
|
— |
|
|
132,273,773 |
||||||
|
Property and equipment, net |
|
— |
|
34,188 |
|
|
— |
|
|
34,188 |
||||||
|
Goodwill |
|
— |
|
— |
|
|
18,486,810 |
|
(A) |
|
18,486,810 |
|||||
|
Long-term portion of prepaid expenses |
|
— |
|
129,740 |
|
|
— |
|
|
129,740 |
||||||
|
Total assets |
$ |
132,277,079 |
$ |
9,250,036 |
|
$ |
30,492,810 |
|
$ |
172,019,925 |
||||||
|
|
|
|
|
|
|
|||||||||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
||||||||||
|
Current liabilities: |
|
|
|
|
|
|
||||||||||
|
Accounts payable and accrued expenses |
$ |
— |
$ |
567,633 |
|
$ |
— |
|
$ |
567,633 |
||||||
|
Accrued interest – related party |
|
— |
|
21,651 |
|
|
— |
|
|
21,651 |
||||||
|
Advance payable to related party |
|
— |
|
100 |
|
|
— |
|
|
100 |
||||||
|
Notes payable – related party |
|
— |
|
500,000 |
|
|
— |
|
|
500,000 |
||||||
|
Total current liabilities |
|
— |
|
1,089,384 |
|
|
— |
|
|
1,089,384 |
||||||
|
Convertible note, net of debt discount |
|
|
|
|
|
|
12,006,000 |
|
(C) |
|
12,006,000 |
|||||
|
Total liabilities |
|
— |
|
1,089,384 |
|
|
12,006,000 |
|
|
13,095,384 |
||||||
|
|
|
|
|
|
|
|||||||||||
|
Stockholders’ equity: |
|
|
|
|
|
|
||||||||||
|
Preferred stock |
|
— |
|
— |
|
|
1,495,403 |
|
(A) |
|
— |
|||||
|
|
|
|
|
(1,495,403 |
) |
(D) |
|
|||||||||
|
Common stock |
|
10 |
|
125,760 |
|
|
149,705 |
|
(B) |
|
1,645,108 |
|||||
|
|
|
|
|
(125,770 |
) |
(B) |
|
|||||||||
|
|
|
|
|
1,495,403 |
|
(D) |
|
|||||||||
|
Additional paid-in capital |
|
82,969,083 |
|
17,272,661 |
|
|
25,002,363 |
|
(A) |
|
107,971,446 |
|||||
|
|
|
|
|
(17,272,661 |
) |
(B) |
|
|||||||||
|
Retained earnings (deficit) |
|
49,307,986 |
|
(9,237,769 |
) |
|
9,237,769 |
|
(B) |
|
49,307,986 |
|||||
|
Total stockholders’ equity |
|
132,277,079 |
|
8,160,652 |
|
|
18,486,810 |
|
|
158,924,541 |
||||||
|
Total liabilities and stockholders’ equity |
$ |
132,277,079 |
$ |
9,250,036 |
|
$ |
30,492,810 |
|
$ |
172,019,925 |
||||||
(A) The purchase consideration is measured as the fair value of the 9.1% equity interest retained by the original APUS stockholders. Using the quoted market price of $1.78 of APUS at September 30, 2025, the total consideration is $26,647,462 based on 14,970,484 common shares held by APUS. Under ASC 805-30-30-1, the excess of this price over the $8,160,652 fair value of APUS’s net assets results in the recognition of $18,486,810 in pro forma goodwill.
(B) The historical equity of APUS is eliminated to reflect MindWave as the accounting acquirer. Under ASC 805-40-45-1, the pre-acquisition retained earnings (deficit) and common stock of APUS are reversed in their entirety, as only MindWave’s historical earnings carry forward. These balances are replaced by the “deemed issuance” of 14,970,484 shares at their $1.78 fair value, with the par value credited to preferred stock and the remainder to additional paid-in capital.
109
(C) On December 1, 2025, the Company entered into a Securities Purchase Agreement with an institutional investor for senior unsecured convertible notes totaling $12,900,000 with an 8% original issue discount. At closing, $10,875,000 was funded, with an additional $2,175,000 expected upon effectiveness of the resale registration statement. The adjustment above reflects both the proceeds from the first tranche and expected proceeds upon effectiveness. A debt discount of $1,044,000 was recorded, yielding net proceeds of $12,006,000. The notes bear no interest unless in default, mature 12 months from issuance at par, and are convertible at 80% of the lowest five-day VWAP, subject to monthly limits.
(D) In connection with the merger, the Company issued preferred stock, which has not yet been converted. Upon shareholder approval of the transaction, effective once the information statement is completed and distributed to APUS shareholders, each share of preferred stock will be convertible into common stock in accordance with the governing agreements. For pro forma purposes, the preferred stock has been reflected as converted into common stock in adjustment A.
110
notes to the unaudited pro forma financial information
Basis of Presentation
The unaudited pro forma consolidated financial information has been prepared to illustrate the effect of the merger between MindWave Innovations Inc. (“MindWave”) and APUS, which was consummated on December 1, 2025. For accounting purposes, the merger is treated as a reverse acquisition in accordance with ASC 805-40. Although APUS is the legal acquirer and the surviving reporting entity, MindWave is designated as the accounting acquirer because its former stockholders hold a 90.9% controlling interest in the combined company. Consequently, the historical financial statements of MindWave become the historical financial statements of the consolidated company, and the assets and liabilities of APUS are recorded at their acquisition-date fair values.
The fiscal year end of MindWave is March 31, 2025, which was used as the basis of presentation in the accompanying pro forma financial statements.
Purchase Price Allocation
The purchase consideration is measured based on the “deemed issuance” of equity interests as prescribed by ASC 805-40-30-2. This represents the fair value of the 9.1% equity interest retained by the original APUS stockholders. Based on the 14,970,484 shares of APUS common stock outstanding at the closing date and the quoted market price of $1.78 per share (a Level 1 input under ASC 820), the total purchase consideration is $26,647,462.
Pro Forma Adjustments
The pro forma adjustments reflect the elimination of APUS’s historical equity balances and the recognition of the new capital structure. Specifically, adjustments were made to remove APUS’s historical common stock, additional paid-in capital, and retained earnings, as MindWave’s historical earnings are the only ones carried forward under ASC 805-40-45-1. Additional adjustments include the recording of the par value of the shares issued in the merger. These adjustments are intended to present the financial position and results of operations as if the transaction had occurred at the beginning of each respective period for the statement of operations and as of the most recent period-end for the balance sheet.
Earnings Per Share (EPS)
Pro forma earnings per share is calculated using the weighted-average number of shares outstanding, giving effect to the exchange ratio established in the Merger Agreement. In accordance with reverse acquisition guidance, the number of shares used to calculate historical EPS has been retroactively recast to reflect the 90.9% ownership stake held by MindWave shareholders. This ensures that the earnings per share data is comparable across all periods presented and reflects the impact of the 14,970,484 shares retained by APUS shareholders as if those shares were outstanding throughout the entire duration of the periods reported.
Consideration Transferred
|
Number shares to be retained by APUS stockholders |
|
14,970,484 |
|
|
Quoted price of APUS |
$ |
1.78 |
|
|
Purchase Consideration |
$ |
26,647,462 |
|
|
|
|||
|
Book value of net assets of APUS |
|
8,160,652 |
|
|
Goodwill |
$ |
18,486,810 |
111
THE PREFERRED STOCK CONVERSION
Overview
Prior to the entry into the Merger Agreement, the Company was authorized to issue up to 10,000,000 shares of preferred stock. Contemporaneously with the execution and delivery of the Merger Agreement, on December 1, 2025, the Company caused to be filed with the DE SOS a Certificate of Designation, setting forth the preferences, rights, and limitations of the Company preferred stock, as amended by the filing of a Certificate of Correction to the Certificate of Designation on December 10, 2025 (the “Certificate of Designation”). The original Certificate of Designation is attached as Annex E and the Certificate of Correction to the Certificate of Designation is attached as Annex F.
The Certificate of Designation designated 7,477,017 shares of the Company’s preferred stock as “Series A Convertible Preferred Stock”, which (i) shall have no voting rights, (ii) shall be entitled to receive the same dividend or distribution as if the shares of Company preferred stock had been converted into Company Common Stock immediately prior to the record date for such dividend or distribution, (iii) shall be entitled to share pro rata with the holders of Company Common Stock in any distribution of the remaining assets of the Company, (iv) is not convertible at the election of the holder, and (v) effective as of 5:00 p.m. Eastern time on the date that is the third business day following the later of (i) the Action Effective Time, which is the date on which the approval of the Preferred Stock Conversion (each share of Company Common Stock issued in connection with the Preferred Stock Conversion, a “Conversion Share”) in accordance with the listing rules of the NYSE American, is effective, and (ii) the date on which the NYSE American has approved any required new listing application, including any resulting from a change in control or Reverse Merger (as defined in Section 341 of the NYSE American Company Guide), such that (A) the Company satisfies all applicable continuing listing requirements of the NYSE American (or has been granted a grace period therefrom), (B) the Company has not received any notice of non-compliance from the NYSE American, and (C) the Conversion Shares have been approved for listing on the NYSE American, each share of Company Preferred Stock then outstanding shall automatically, and without any action required by the holder thereof, convert into a number of shares of Company Common Stock equal to the Conversion Ratio (as defined below).
The “Conversion Ratio” for each share of Company Preferred Stock shall be twenty (20) shares of Company Common Stock issuable upon the Preferred Stock Conversion of each share of Company Preferred Stock, subject to adjustment as provided in the Certificate of Designation.
At the Effective Time, (i) each share of MindWave common stock issued and outstanding immediately prior to the Effective Time shall be canceled and converted into the right to receive a portion of the Merger Consideration, consisting of shares of Company Preferred Stock; and (ii) each holder of such shares shall receive, for each share of MindWave Common Stock held immediately prior to the Effective Time, a pro rata portion of the Merger Consideration, allocated as follows: (A) a number of duly authorized, validly issued, fully paid and nonassessable shares of Company Common Stock, such that the aggregate number of shares of Company Common Stock issued to all holders of MindWave Common Stock shall equal 0% of the total number of shares of Company Common Stock issued and outstanding as of the date of the Merger Agreement (the “Common Stock Cap”), with each holder’s allocation rounded down to the nearest whole share; and (B) a number of duly authorized, validly issued, fully paid and nonassessable shares of Company Preferred Stock, such that, immediately following the Effective Time, the holders of MindWave Common Stock collectively hold, on an as-converted to Company Common Stock basis, 90.9% of the total issued and outstanding equity securities of the Company (exclusive of the Company Common Stock issued pursuant to clause (A) and calculated on a fully diluted basis). The shares of Company Common Stock and Company Preferred Stock issued to holders of MindWave Common Stock pursuant to the Merger Agreement, on an as-converted and fully diluted basis, shall collectively represent 90.9% of the equity capital of the Company as of the Closing. For purposes of the Merger Agreement, the shares of Company Common Stock, Company Preferred Stock, and MindWave Common Stock issued pursuant to the Merger Agreement are collectively referred to as the “Merger Consideration.”
Additional information on the Certificate of Designation is contained in the Company’s Current Reports on Form 8-K filed with the SEC on December 2, 2025 and December 10, 2025.
NYSE American Requirements and the Necessity of Stockholder Approval
Our Common Stock is currently listed on the NYSE American, and therefore we are subject to the rules of that exchange (the “NYSE American Rules”). Section 713 of the NYSE American Rules requires stockholder approval prior to the issuance of securities in connection with a merger, acquisition, or other transaction where the issuance or potential issuance of common stock (or securities convertible into or exercisable for common stock) could result in an increase in the number of outstanding common shares of 20%.
112
Upon the effectiveness of the Preferred Stock Conversion, the total number of shares of Common Stock issued will exceed 19.9% of the number of shares of Common Stock outstanding. Accordingly, pursuant to Section 713 of the NYSE American Rules, stockholder approval of the Preferred Stock Conversion is required. On December [—], 2025, the Consenting Stockholders, in accordance with Section 228 of the DGCL, executed the Written Consent approving the Preferred Stock Conversion.
Effect on Current Stockholders
The Preferred Stock Conversion will increase the number of shares of our Common Stock outstanding and will result in dilution of the ownership percentage of our existing stockholders. Because the number of shares of Common Stock issuable upon the Preferred Stock Conversion is fixed under the Certificate of Designation (subject to customary anti-dilution adjustments and administrative rounding), the precise dilutive effect will depend on the number of shares outstanding at the Effective Date.
Stockholder Approval of the Preferred Stock Conversion
The approval of the Preferred Stock Conversion, including for purposes of NYSE American rules, required the affirmative approval of a majority of the holders of the outstanding shares of the Company’s Common Stock that were entitled to approve the Preferred Stock Conversion.
As of the Record Date, the Company had [—] shares of Common Stock issued and outstanding and the Consenting Stockholder held [—] shares of Common Stock as of the Record Date, representing approximately [—]% of the voting power of all shares of the Company’s Common Stock. The Consenting Stockholders approved the Preferred Stock Conversion upon execution of the Written Consent on [—], 2025, following execution of the Merger Agreement.
This Information Statement is first being mailed on or about [—], 2026, to the Company’s stockholders of record as of the Record Date. The Preferred Stock Conversion shall be effective on or about [—], 2026, or approximately 20 days after we mail this Information Statement.
Notice Pursuant to Section 228 of the DGCL
Under the DGCL and the Company’s governing documents, the Consenting Stockholders approved the Preferred Stock Conversion. No further vote or action by any other stockholders is required, and no meeting is being held. This Information Statement is being furnished solely to satisfy the notice requirements of DGCL §228.
However, in accordance with the waiting period required by Rule 14c-2 of the Exchange Act, the Preferred Stock Conversion will not become effective until at least twenty (20) calendar days after the Company sends this Information to its stockholders of record as of the Record Date.
113
THE NOTES CONVERSION AND ISSUANCE
Overview
On December 1, 2025, the Company entered into a Securities Purchase Agreement, attached as Annex B, as amended on December 8, 2025, by that certain Amendment No. 1 to Securities Purchase Agreement (the “Securities Purchase Agreement”), attached as Annex C, with an institutional investor (the “Investor”), pursuant to which the Company agreed to issue senior convertible notes (the “Notes”) in an aggregate principal amount of up to $120,900,000 at an 8% original issue discount (the “Note Financing”), over a 24-month period (the “Commitment Period”).
The Notes bear no interest unless an event of default occurs. The Notes constitute senior unsecured obligations of the Company. The Company is required to file a resale registration statement on Form S-1 (the “Resale Registration Statement”) within 45 days of closing and have it declared effective within 75 days (or 120 days in the event of a full SEC review), subject to certain extensions, registering the shares of Company Common Stock issuable pursuant to the terms of the Notes. Delays beyond these deadlines will result in liquidated damages of 1% of the redemption value for each 30-day period.
In connection with the closing of the Securities Purchase Agreement, $10,875,000 of the principal amount of the Notes shall be made available to the Company, and an additional $2,175,000 shall be funded upon the effectiveness of the Resale Registration Statement. The Investor shall have the right, at its sole discretion, to purchase up to an aggregate of $13,075,000 of such additional Notes in one or more closings. The Investor shall have the ability to purchase, subject to mutual consent of the parties, any amount remaining under the Notes. The Notes shall mature on the 12-month anniversary of their issuance at 100% of the face value. The Investor has the right to convert the notes at 80% of the lowest daily volume weighted average price during the 5-trading day period ending and including the date of conversion (the “Conversion Price”). Monthly conversions using the Conversion Price shall be limited to the greater of (i) 20% of the aggregate of the daily traded value during such calendar month period, and (ii) $2.25 million.
The Notes include customary negative and affirmative covenants for transactions of this type. The investor has agreed to a no net short provision, subject to certain carve-outs. The Investor also has the right to participate in up to 30% of any subsequent financing until the later of six months after the Commitment Period or the Notes’ maturity date.
The Securities Purchase Agreement provides that the Note shall not be fully convertible until the Company receives stockholder approval, and upon such approval, the Notes shall be convertible at the Conversion Price described above.
In connection with the Securities Purchase Agreement, on January 8, 2025, the Company entered into that certain Amendment and Exchange Agreement, attached as Annex D, which amends certain terms of the Securities Purchase Agreement, by authorizing a new series of senior secured convertible notes in the aggregate original principal amount of $120,900,000, in exchange of the Notes.
Additional information on the Securities Purchase Agreement is contained in the Company’s Current Reports on Form 8-K filed with the SEC on December 2, 2025 and December 10, 2025.
NYSE American Requirements and the Necessity of Stockholder Approval
Section 713(a) of the NYSE American Company Guide requires an issuer to obtain stockholder approval as a prerequisite to approval of an application to list additional shares whenever the issuer’s sale, issuance, or potential issuance of common stock (or securities convertible into or exercisable for common stock) equals or exceeds 20% of the issuer’s pre-transaction outstanding shares at a price below the “Minimum Price”, as defined in Section 713(c). Shares of common stock issuable upon the exercise or conversion of warrants, options, debt instruments, preferred stock or other equity securities issued or granted in such non-public offerings will be considered shares issued in such a transaction in determining whether such 20% limit has been reached, subject to certain exceptions.
The Securities Purchase and the Notes provide that the Notes are not convertible and the Company shall not issue any shares of Common Stock upon conversion of the Notes or otherwise, if such issuance would exceed the aggregate number of shares of Common Stock permitted under the rules or regulations of NYSE American (such maximum number of shares, the “Exchange Cap”).
Therefore, the Consenting Stockholders therefore approved the issuance of the number of shares of Common Stock in excess of the Exchange Cap in order to comply with the NYSE American rules.
114
Effect of Stockholder Approval on the Exchange Cap
By obtaining stockholder approval of the issuance of such number of shares of Common Stock in excess the Exchange Cap, we now have the option to issue the maximum number of shares of Common Stock issuable under the Note, which would exceed 19.99% of our issued and outstanding shares of Common Stock as of the date we executed the Securities Purchase Agreement. The rights or privileges of existing stockholders will not be affected, except that the economic and voting interests of each of our existing stockholders will be diluted should we issue any shares of Common Stock pursuant to the Notes. Although the number of shares of our Common Stock that our existing stockholders own will not decrease, the shares of our Common Stock owned by our existing stockholders will represent a smaller percentage of our total outstanding shares of our Common Stock after any such issuance.
Stockholder Approval of the Notes Conversion
The approval of the Notes Conversion, including for purposes of NYSE American rules, required the affirmative approval of a majority of the holders of the outstanding shares of the Company’s Common Stock that were entitled to approve the Notes Conversion.
As of the Record Date, the Company had [—] shares of Common Stock issued and outstanding and the Consenting Stockholder held [—] shares of Common Stock as of the Record Date, representing approximately [—]% of the voting power of all shares of the Company’s Common Stock. The Consenting Stockholders approved the Notes Conversion upon execution of the Written Consent on [—], 2025, following execution of the Merger Agreement.
This Information Statement is first being mailed on or about [—], 2026, to the Company’s stockholders of record as of the Record Date. The Notes Conversion shall be effective on or about [—], 2026, or approximately 20 days after we mail this Information Statement.
Notice Pursuant to Section 228 of the DGCL
Under the DGCL and the Company’s governing documents, the Consenting Stockholders approved the Notes Conversion. No further vote or action by any other stockholders is required, and no meeting is being held. This Information Statement is being furnished solely to satisfy the notice requirements of DGCL §228.
However, in accordance with the waiting period required by Rule 14c-2 of the Exchange Act, the Notes Conversion will not become effective until at least twenty (20) calendar days after the Company sends this Information to its stockholders of record as of the Record Date.
115
Overview
On December [—], 2025, the Consenting Stockholders executed the Written Consent approving a reverse stock split of the Company’s Common Stock at a ratio of one-for-ten (the “Reverse Stock Split”) and a change in the par value per share of the Company’s Common Stock from $0.01 to $0.001 (the “Change in Par Value”). The Reverse Stock Split and the Change in Par Value were previously approved by the Board of Directors and recommended for stockholder approval. The Reverse Stock Split and the Change in Par Value will be effected by filing a Certificate of Amendment to the Company’s Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Delaware (the “Certificate of Amendment”), attached as Annex G, and will become effective at the time specified therein.
Purpose of the Reverse Stock Split
The primary purpose of the Reverse Stock Split is to increase the per share trading price of the Company’s Common Stock. The Consenting Stockholders believe that increase the market price of the Company’s Common Stock may improve the Company’s ability to satisfy applicable listing or continued listing requirements, broaden the pool of potential investors, enhance the marketability of the Company’s Common Stock, and reduce volatility associated with a low trading price.
Upon the effectiveness of the Reverse Stock Split, each ten (10) shares of the Company’s Common Stock will automatically be combined into one issued and outstanding share of Common Stock, and the par value per share of the Company’s common stock will be reduced from $0.01 to $0.001.
Effects of the Reverse Stock Split
The Reverse Stock Split will affect all stockholders uniformly and will not affect any stockholder’s percentage ownership interest in the Company, except to the extent that the treatment of fractional shares may result in small changes. The Reverse Stock Split will not change the number of authorized shares of Common Stock, the rights and preferences of the Common Stock, or the percentage ownership of any stockholder other than with respect to fractional share treatment.
The Reverse Stock Split will not change the rights, preferences, or terms of the Company’s Common Stock. Following the Reverse Stock Split, each share of Common Stock will have the same voting rights and rights to dividends and distributions and will be identical in all other respects to the Common Stock currently authorized. All shares of Common Stock outstanding following the Reverse Stock Split will remain fully paid and non-assessable.
Following the Reverse Stock Split, the market price of the Common Stock may be lower than the pre-split price multiplied by the Reverse Split Ratio. In addition, a reduction in the number of shares outstanding may reduce the trading liquidity of the Common Stock, which could adversely affect its market value.
Upon the effective of the Reverse Stock Split, the par value per share of the Company’s Common Stock will be reduced from $0.01 to $0.001. The reduction in par value will not affect the rights, preferences, or privileges of the Company’s Common Stock.
The Reverse Stock Split will not change the total number of shares of Common Stock that the Company is authorized to issue under its Charter. As a result, the number of authorized but unissued shares of Common Stock will increase, which may be used by the Company for future financings, acquisitions, equity compensation, or other corporate purposes without further stockholder approval.
Fractional Shares
No fractional shares will be issued in connection with the Reverse Stock Split. In lieu of fractional shares, each stockholder otherwise entitled to receive a fractional share will receive one whole share of Common Stock.
116
Effect of the Reverse Stock Split on Equity Incentive Plans, Options, and Warrants
Proportionate adjustments will be made to the number of shares of Common Stock issuable upon the exercise or conversion of the Company’s outstanding stock options, restricted stock awards, warrants, and convertible securities, as well as to their respective exercise or conversion prices, in accordance with their terms and the Reverse Stock Split ratio.
Stockholder Approval of the Reverse Stock Split
Under the DGCL and the Charter, the Consenting Stockholders approved the Reverse Stock Split and the Change in Par Value by written consent pursuant to DGCL §228. No further vote or action by any other stockholders is required, and no meeting is being held. This Information Statement is being furnished solely to satisfy the notice requirements of DGCL §228 and Section 14© of the Exchange Act.
However, in accordance with the waiting period required by Rule 14c-2 of the Exchange Act, the Reverse Stock Split will not become effective until at least twenty (20) calendar days after the Company sends this Information to its stockholders of record as of the Record Date.
No Appraisal or Dissenter’s Rights
No dissenters’ or appraisal rights are available under the DGCL or the Company’s organizational documents in connection with the approval of the Reverse Stock Split.
117
Overview
On December 1, 2025, the Consenting Stockholders approved an amendment to the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan (the “2024 Plan”), attached as Annex H, to increase the aggregate number of shares of Company Common Stock authorized for issuance under the 2024 Plan to 2,096,679 Common Shares (the “2024 Plan Share Increase”).
The 2024 Plan Share Increase was necessary as the 2024 Plan did not have a sufficient number of authorized shares to be issued under the 2024 Plan in connection with the transactions contemplated by the Merger Agreement. The Board of Directors did not make any further changes to the 2024 Plan other than effecting the 2024 Plan Share Increase.
Key Provisions of the 2024 Plan
The following is a summary of the principal features of the 2024 Plan, which does not purport to be a complete description of all of the provisions of the 2024 Plan. This summary is qualified in its entirety by reference to the full text of the 2024 Plan, which is attached as Annex I.
On September 18, 2024, the Company adopted the 2024 Plan for its employees. The purposes of the 2024 Plan are to provide additional incentives to selected employees, directors and independent contractors of, and consultants to, the Company or its affiliates, to strengthen their commitment, motivate them to faithfully and diligently perform their responsibilities and to attract and retain competent and dedicated persons who are essential to the success of our business and whose efforts will impact our long-term growth and profitability.
Federal Income Tax Consequences of 2024 Plan Awards
The following is a general summary of the material U.S. federal income tax consequences of the grant and exercise and vesting of awards under the 2024 Plan and the disposition of shares acquired pursuant to the exercise or settlement of such awards and is intended to reflect the current provisions of the Code and the regulations thereunder. This summary is not intended to be a complete statement of applicable law, nor does it address foreign, state, local and payroll tax considerations, and does not describe federal taxes other than income taxes. Moreover, the U.S. federal income tax consequences to any participant may differ from those described herein by reason of, among other things, the particular circumstances of such participant. The following is not to be considered tax advice to any persons who may be participants in the 2024 Plan, and any such persons are advised to consult with their own tax counsel.
Stock Options. The 2024 Plan authorizes the Compensation Committee to grant both options that are “qualified,” meaning they are intended to satisfy the requirements of Section 422 of the Code (also referred to as incentive stock options), and options that are “non-qualified,” meaning they are not intended to satisfy the requirements of Section 422 of the Code.
Holders of incentive stock options generally will not incur federal income tax liability at the time of grant or upon exercise of qualified incentive stock options, provided that they meet certain employment criteria and satisfy holding period requirements. However, the spread at exercise will be an “item of tax preference,” which may give rise to “alternative minimum tax” liability for the taxable year in which the exercise occurs. For treatment of an option as an incentive stock option, shares of Common Stock acquired through the exercise of an incentive stock option cannot be disposed of before the later of (i) two years from the date of grant of the option, or (ii) one year from the date of exercise. If the holder satisfies the holding period requirements, the difference between the exercise price and the amount realized upon a subsequent disposition of the shares will constitute long-term capital gain or loss, as the case may be. Assuming both holding periods are satisfied, no deduction will be allowed to us for federal income tax purposes in connection with the grant or exercise of the incentive stock option. If a holder disposes of such shares without meeting the holding period requirements, the participant generally will realize taxable income at the time of such disposition equal to the difference between the exercise price and the lesser of the fair market value of the shares on the date of exercise or the amount realized on the subsequent disposition of the shares, and that amount will generally be deductible by us for federal income tax purposes, subject to the possible limitations on deductibility under Sections 280G and 162(m) of the Code for compensation paid to executives designated in those sections. Finally, if
118
an incentive stock option becomes first exercisable in any one year for shares having an aggregate value more than $100,000 (based on the grant date value), the portion of the incentive stock option in respect of those excess shares will be treated as a non-qualified stock option for federal income tax purposes.
No income will be realized by a participant upon grant of a non-qualified option. Upon the exercise of a non-qualified stock option, the participant will recognize ordinary income in an amount equal to the excess, if any, of the fair market value of the underlying exercised shares over the option exercise price paid at the time of exercise. The Company will be able to deduct this same amount for U.S. federal income tax purposes, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Stock Appreciation Rights. No income will be realized by a participant upon grant of a Stock Appreciation Rights (“SAR”). Upon settlement of a SAR, the participant will recognize ordinary income in an amount equal to the fair market value of the payment received in respect of the SAR. The Company will be able to deduct this same amount for U.S. federal income tax purposes, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Restricted Stock. A participant will not be subject to tax at ordinary income rates upon the grant of an award of restricted stock unless the participant otherwise elects to be taxed at the time of grant pursuant to Section 83(b) of the Code. On the date an award of restricted stock becomes transferable or is no longer subject to a substantial risk of forfeiture, the participant will have taxable income equal to the difference between the fair market value of the shares on that date over the amount the participant paid for such shares, if any, unless the participant made an election under Section 83(b) of the Code to be taxed at the time of grant. If the participant made an election under Section 83(b), the participant would have taxable compensation at the time of grant equal to the difference between the fair market value of the shares on the date of grant over the amount the participant paid for such shares, if any. (Special rules apply to the receipt and disposition of restricted shares received by officers and directors who are subject to Section 16(b) of the Exchange Act.) The Company generally will be able to deduct a share-based award, in the same amount and in the same tax year as it is recognized by the participant, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Restricted Stock Units. A participant will not be subject to tax at ordinary income rates upon the grant of a restricted stock unit award. Rather, upon the delivery of shares or cash pursuant to a restricted stock unit award, the participant will have taxable income equal to the fair market value of the number of shares (or the amount of cash) the participant receives with respect to the award (less any amount paid by the participant for such restricted stock unit(s)). The Company will be able to deduct the amount of taxable compensation to the participant for U.S. federal income tax purposes, but the deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Stock Bonus Awards. Generally, participants will recognize taxable income at the time of settlement of stock bonus awards (with the amount of income recognized generally being equal to the fair market value of any shares delivered under the award). The Company will be able to deduct the amount of taxable compensation to the participant for U.S. federal income tax purposes, but the deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Performance Compensation Awards. No income generally will be recognized upon the grant of performance compensation awards. Upon payment in respect of the earn-out of performance compensation awards, the recipient generally will be required to include as taxable ordinary income in the year of receipt an amount equal to the amount of cash received and the fair market value of any unrestricted shares of our Common Stock received. The Company will be able to deduct the amount of taxable compensation to the participant for U.S. federal income tax purposes, but the deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Tax Deductibility of Compensation Provided Under the 2024 Plan. When a participant recognizes ordinary compensation income as a result of an award granted under the 2024 Plan, the Company may be permitted to claim a federal income tax deduction for such compensation, subject to various limitations that may apply under applicable law (see discussion of Section 162(m) and 280G below, for two examples of limitations that may apply).
Section 162(m). Compensation of persons who are “covered employees” is subject to the tax deduction limits of Section 162(m) of the Code. Prior to the enactment of the Tax Cuts and Jobs Act (the “TCJA”) on December 22, 2017, awards that qualified as “performance-based compensation” were exempt from Section 162(m), thereby permitting us
119
to claim the full federal tax deduction otherwise allowed for such compensation. This exception was eliminated by the TCJA. However, pending further guidance, this exception may still be available under state law allowing us to claim the full state deduction otherwise allowed for such compensation. In addition, the TCJA includes a transition rule under which the change described above will not apply to compensation payable pursuant to a written binding contract that was in effect on November 2, 2017 and is not materially modified after that date. To the extent applicable to our existing contracts and awards, our Compensation Committee may avail itself of this transition rule.
Section 280G. To the extent that compensation provided under the 2024 Plan may be deemed to be contingent upon a change in control, a portion of such compensation may be non-deductible by the Company under Section 280G of the Code and may be subject to a 20% excise tax imposed on the recipient of the compensation.
Section 409A. Section 409A of the Code imposes certain restrictions upon the payment of nonqualified deferred compensation. To the extent applicable, it is intended that the 2024 Plan and any grants made thereunder comply with the provisions of Section 409A of the Code, so that the income inclusion provisions of Section 409A(a)(1) of the Code do not apply to the participants. The 2024 Plan and any grants made thereunder will be administered in a manner consistent with this intent.
Securities Available Under the 2024 Plan
Awards
The 2024 Plan allows the Company to make equity and equity-based incentive awards to officers, employees, directors, consultants, and advisors. The Board of Directors anticipates that providing such persons with a direct stake in the Company will assure a closer alignment of the interests of such individuals with those of the Company and its stockholders, thereby stimulating their efforts on the Company’s behalf and strengthening their desire to remain with the Company.
The 2024 Plan provides for the grant of non-qualified stock options, incentive stock options, stock appreciation rights, restricted stock, restricted stock units, stock bonus awards, and performance compensation awards. All awards will be set forth in an award agreement which will detail all terms and conditions of the awards, including any applicable vesting and payment terms and post-termination exercise limitations.
A brief description of each award type follows.
• Non-Qualified Stock Options means the right to purchase shares pursuant to terms and conditions that are not intended to be, or do not qualify as, an Incentive Stock Options;
• Incentive Stock Options means the right to purchase shares pursuant terms and conditions that are intended to qualify as, and that satisfy the requirements applicable to, an incentive equity option within the meaning of Code Section 422 of the United States Internal Revenue Code of 1986, as amended;
• Stock Appreciation Rights means a right, designated as an SAR, to receive the appreciation in the fair market value of shares;
• Restricted Stock means an award of shares subject to vesting conditions;
• Restricted Stock Units shall mean a right to receive shares or cash upon vesting;
• Stock Bonus Awards means unrestricted common stock, or other awards denominated in common stock, either alone or in tandem with other awards; and
• Performance Compensation Awards means an award granted to a participant that entitles the participant to delivery of shares or cash upon achievement of performance goals.
1,000,000 shares of common stock were initially reserved for the issuance of awards under the 2024 Plan (the “Initial Limit”). The Initial Limit was increased pursuant to the 2024 Plan Share Increase amendment to 2,096,679 shares of common stock (the “Subsequent Limit”). The Subsequent Limit is subject to adjustment in the event of a reorganization, recapitalization, reclassification, stock split, stock dividend, reverse stock split or other similar change in the Company’s capitalization. The maximum aggregate number of shares of common stock of the Company that may be issued upon exercise of incentive stock options under the 2024 Plan shall not exceed the Subsequent Limit, as adjusted. Shares underlying any awards under the 2024 Plan that are forfeited, cancelled, held back upon exercise of an option or
120
settlement of an award to cover the exercise price or tax withholding, satisfied without the issuance of stock or otherwise terminated (other than by exercise) will be added back to the shares available for issuance under the 2024 Plan and, to the extent permitted under Section 422 of the Code and the regulations promulgated thereunder, the shares that may be issued as incentive stock options
The 2024 Plan is currently administered by a committee of at least two people as the Board of Directors may appoint to administer the 2024 Plan or, if no such committee has been appointed by the Board of Directors, the Board of Directors, pursuant to the terms of the 2024 Plan (the “Committee”). The plan administrator, which initially will be the Committee, has full power to select, from among the individuals eligible for awards, the individuals to whom awards will be granted, to make any combination of awards to participants, and to determine the specific terms and conditions of each award, subject to the provisions of the 2024 Plan. The plan administrator may delegate to a committee consisting of one or more officers of the Company, the authority to awards to individuals who are not subject to the reporting and other provisions of Section 16 of the Exchange Act and not members of the delegated committee, subject to certain limitations and guidelines.
Persons eligible to participate in the 2024 Plan will be officers, employees, non-employee directors, consultants, and advisors of the Company and its subsidiaries as selected from time to time by the plan administrator in its discretion. As of the date of this Information Statement, approximately [—] individuals are eligible to participate in the 2024 Plan, which includes approximately [—] officers, no employees who are not officers, [—] non-employee directors, and [—] consultants/independent contractors.
Options
The 2024 Plan permits the granting of both options to purchase common stock of the Company intended to qualify as incentive stock options under Section 422 of the Code and options that do not so qualify. Options granted under the 2024 Plan will be non-qualified options if they fail to qualify as incentive stock options or exceed the annual limit on incentive stock options. Incentive stock options may only be granted to employees of the Company and its subsidiaries. Non-qualified options may be granted to any persons eligible to receive awards under the 2024 Plan. The option exercise price of each option will be determined by the plan administrator but generally may not be less than 100% of the fair market value of the common stock of the Company on the date of grant or, in the case of an incentive stock option granted to a ten percent stockholder, 110% of such share’s fair market value. The term of each option will be fixed by the plan administrator and may not exceed ten years from the date of grant. The plan administrator will determine at what time or times each option may be exercised, including the ability to accelerate the vesting of such options.
Upon exercise of options, the option exercise price must be paid in full either in cash, by certified or bank check or other instrument acceptable to the plan administrator or by delivery (or attestation to the ownership) of shares of common stock of the Company that are beneficially owned by the optionee free of restrictions or were purchased in the open market. Subject to applicable law, the exercise price may also be delivered by a broker pursuant to irrevocable instructions to the broker from the optionee. In addition, the plan administrator may permit non-qualified options to be exercised using a “net exercise” arrangement that reduces the number of shares issued to the optionee by the largest whole number of shares with fair market value that does not exceed the aggregate exercise price.
Stock Appreciation Rights
The plan administrator may award stock appreciation rights subject to such conditions and restrictions as it may determine. Stock appreciation rights entitle the recipient to shares of common stock of the Company, or cash, equal to the value of the appreciation in the Company’s stock price over the exercise price. The exercise price generally may not be less than 100% of the fair market value of common stock of the Company on the date of grant. The term of each stock appreciation right will be fixed by the plan administrator and may not exceed ten years from the date of grant. The plan administrator will determine at what time or times each stock appreciation right may be exercised, including the ability to accelerate the vesting of such stock appreciation rights.
Restricted Stock and Restricted Stock Units
The plan administrator may award restricted shares of common stock of the Company and restricted stock units to participants subject to such conditions and restrictions as it may determine. These conditions and restrictions may include the achievement of certain performance goals and/or continued employment with the Company through a specified vesting period. The plan administrator may also grant shares of common stock of the Company that are free
121
from any restrictions under the 2024 Plan. Unrestricted stock may be granted to participants in recognition of past services or for other valid consideration and may be issued in lieu of cash compensation due to such participant. The plan administrator may grant dividend equivalent rights to participants that entitle the recipient to receive credits for dividends that would be paid if the recipient had held a specified number of shares of common stock of the Company.
Stock Bonus Awards
The plan administrator may issue unrestricted common stock, or other awards denominated in common stock, under the 2024 Plan to participants, either alone or in tandem with other awards, in such amounts as the plan administration shall from time to time in its sole discretion determine.
Performance Compensation Awards
The plan administrator may grant awards under the 2024 Plan to participants, which may be cash-based, subject to the achievement of certain performance goals, including continued employment with the Company.
Other Material Features of the 2024 Plan
The 2024 Plan requires the plan administrator to make appropriate adjustments to the number of shares of common stock that are subject to the 2024 Plan, to certain limits in the 2024 Plan, and to any outstanding awards to reflect stock dividends, stock splits, extraordinary cash dividends and similar events.
Except as set forth in a stock award agreement issued under the 2024 Plan, in the event of (i) a transfer of all or substantially all of the Company’s assets, (ii) a merger, consolidation or other capital reorganization or business combination transaction of the Company with or into another corporation, entity or person, or (iii) the consummation of a transaction, or series of related transactions, in which any person becomes the beneficial owner directly or indirectly, of more than 50% of Company’s then outstanding capital stock, each outstanding stock award (vested or unvested) will be treated as the plan administrator determines, which may include (a) Company’s continuation of such outstanding stock awards (if Company is the surviving corporation); (b) the assumption of such outstanding stock awards by the surviving corporation or its parent; (c) the substitution by the surviving corporation or its parent of new stock options or other equity awards for such stock awards; (d) the cancellation of such stock awards in exchange for a payment to the participants equal to the excess of (1) the fair market value of the shares subject to such stock awards as of the closing date of such corporate transaction over (2) the exercise price or purchase price paid or to be paid (if any) for the shares subject to the stock awards (which payment may be subject to the same conditions that apply to the consideration that will be paid to holders of shares in connection with the transaction, subject to applicable law); or (e) the opportunity for participants to exercise the stock options prior to the occurrence of the corporate transaction and the termination (for no consideration) upon the consummation of such corporate transaction of any stock options not exercised prior thereto.
The 2024 Plan provides that a stock award may be subject to additional acceleration of vesting and exercisability upon or after a “Change in Control” (as defined in the 2024 Plan) as may be provided in the award agreement for such stock award or as may be provided in any other written agreement between the Company or any affiliate and the participant, but in the absence of such provision, no such acceleration will occur.
Participants in the 2024 Plan are responsible for the payment of any federal, state or local taxes that the Company or its subsidiaries are required by law to withhold upon the exercise of options or stock appreciation rights or vesting of other awards. The plan administrator may cause any tax withholding obligation of the Company or its subsidiaries to be satisfied, in whole or in part, by the applicable entity withholding from shares of common stock of the Company to be issued pursuant to an award shares with an aggregate fair market value that would satisfy the withholding amount due. The plan administrator may also require any tax withholding obligation of the Company or its subsidiaries to be satisfied, in whole or in part, by an arrangement whereby a certain number of shares issued pursuant to any award are immediately sold and proceeds from such sale are remitted to the Company or its subsidiaries in an amount that would satisfy the withholding amount due.
122
The 2024 Plan generally does not allow for the transfer or assignment of awards, other than by will or by the laws of descent and distribution or pursuant to a domestic relations order; however, the plan administrator may permit the transfer of non-qualified stock options by gift to an immediate family member, to trusts for the benefit of family members, or to partnerships in which such family members are the only partners.
The plan administrator may amend or discontinue the 2024 Plan and the plan administrator may amend or cancel outstanding awards for purposes of satisfying changes in law or any other lawful purpose, but no such action may materially and adversely affect rights under an award without the holder’s consent. Certain amendments to the 2024 Plan will require the approval of the Company’s stockholders. Generally, without stockholder approval, (i) no amendment or modification of the 2024 Plan may reduce the exercise price of any stock option or the strike price of any stock appreciation right, (ii) the plan administrator may not cancel any outstanding stock option or stock appreciation right where the fair market value of the common stock underlying such stock option or stock appreciation right is less than its exercise price and replace it with a new option or stock appreciation right, another award or cash and (iii) the plan administrator may not take any other action that is considered a “repricing” for purposes of the stockholder approval rules of the applicable securities exchange.
All awards granted under the 2024 Plan will be subject to recoupment in accordance with any clawback policy that Company is required to adopt pursuant to the listing standards of any national securities exchange or association on which Company securities are listed or as is otherwise required by the U.S. Dodd-Frank Wall Street Reform and Consumer Protection Act or other applicable law. In addition, the Board may impose such other clawback, recovery or recoupment provisions in a stock award agreement as the Board determines necessary or appropriate.
No options or stock appreciation rights may be granted under the 2024 Plan after the date that is ten years from the 2024 Plan Effective Date. No awards under the 2024 Plan have been made prior to the date of this Information Statement.
NYSE American Rules and the Necessity for Stockholder Approval
As described above, we are subject to NYSE American rules, including NYSE American Company Guide 711, pursuant to which approval of stockholders is required with respect to the establishment of (or material amendment to) a stock option or purchase plan or other equity compensation arrangement pursuant to which options or stock may be acquired by officers, directors, employees, or consultants, regardless of whether or not such authorization is required by law or by the company’s charter.
Prior to the 2024 Share Plan Increase, [—] shares of the Company’s common stock were available to be issued pursuant to the 2024 Plan. Thus, the 2024 Plan Share Increase resulted in a material increase in the number of shares available for issuance under the 2024 Plan. Given this material increase, the Consenting Stockholders approved the 2024 Plan Share Increase to comply with NYSE American Company Guide 711.
Stockholder Approval of the 2024 Plan Share Increase
The approval of the 2024 Plan Share Increase, including for purposes of NYSE American rules, required the affirmative approval of a majority of the holders of the outstanding shares of the Company’s Common Stock that were entitled to approve the 2024 Plan Share Increase.
As of the Record Date, the Company had [—] shares of Common Stock issued and outstanding and the Consenting Stockholder held [—] shares of Common Stock as of the Record Date, representing approximately [—]% of the voting power of all shares of the Company’s Common Stock. The Consenting Stockholders approved the 2024 Plan Share Increase upon execution of the Written Consent on [—], 2025, following execution of the Merger Agreement.
This Information Statement is first being mailed on or about [—], 2026, to the Company’s stockholders of record as of the Record Date. The 2024 Plan Share Increase shall be effective on or about [—], 2026, or approximately 20 days after we mail this Information Statement.
123
Overview
On December 1, 2025, the Consenting Stockholders approved the Apimeds Pharmaceuticals US, Inc. 2025 Equity Incentive Plan (the “2025 Plan”), attached as Annex J.
The purpose of the 2025 Plan is to advance the interests of the Company, its subsidiaries, affiliates, and stockholders by providing an incentive to attract and retain the best qualified personnel to perform services for the Company, by motivating such individuals to contribute to the growth and the profitability of the Company by aligning such individuals with the interests of the Company’s stockholders, and by rewarding such individuals for their services by tying a signification portion of their compensation to the success of the Company.
Key Provisions of the 2025 Plan
The following is a summary of the principal features of the 2025 Plan, which does not purport to be a complete description of all of the provisions of the 2025 Plan. This summary is qualified in its entirety by reference to the full text of the 2025 Plan, which is attached as Annex J.
In making such determinations, the Board of Directors may take into account the nature of the services rendered by such person, his or her present and potential future contribution to the Company’s success, and such other factors as the Board of Directors in its discretion shall deem relevant. Incentive stock options granted under the 2024 Plan are intended to qualify as “incentive stock options” within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended (the “Code”). Nonqualified (nonstatutory stock options) granted under the 2025 Plan are not intended to qualify as incentive stock options under the Code.
In connection with Merger Agreement, the Consenting Stockholders also approved that a number of shares of the Company’s Common Stock equal to 10% of the Company’s issued and outstanding Common Stock immediately following the closing of the Merger Agreement shall be reserved for issuance under the 2025 Plan. Further, the 2025 Plan includes an evergreen provision providing for the number of shares of the Corporation’s Common Stock reserved for issuance under the 2025 Plan to increase automatically on the first day of each fiscal year during the term of the 2025 Plan, beginning on January 1, 2026, in an amount equal to the lesser of:
• 3% of the total number of shares of Common Stock outstanding on the last day of the preceding fiscal year; or
• such lesser number as may be determined by the Board of Directors (or a committee thereof) prior to the applicable date of increase.
Federal Income Tax Consequences of 2025 Plan Awards
The following is a general summary of the material U.S. federal income tax consequences of the grant and exercise and vesting of awards under the 2025 Plan and the disposition of shares acquired pursuant to the exercise or settlement of such awards and is intended to reflect the current provisions of the Code and the regulations thereunder. This summary is not intended to be a complete statement of applicable law, nor does it address foreign, state, local and payroll tax considerations, and does not describe federal taxes other than income taxes. Moreover, the U.S. federal income tax consequences to any participant may differ from those described herein by reason of, among other things, the circumstances of such participant. The following is not to be considered tax advice to any persons who may be participants in the 2025 Plan, and any such persons are advised to consult with their own tax counsel.
Stock Options. The 2025 Plan authorizes the Compensation Committee to grant both options that are “qualified,” meaning they are intended to satisfy the requirements of Section 422 of the Code (also referred to as incentive stock options), and options that are “non-qualified,” meaning they are not intended to satisfy the requirements of Section 422 of the Code.
Holders of incentive stock options generally will not incur federal income tax liability at the time of grant or upon exercise of qualified incentive stock options, provided that they meet certain employment criteria and satisfy holding period requirements. However, the spread at exercise will be an “item of tax preference,” which may give rise to “alternative minimum tax” liability for the taxable year in which the exercise occurs. For treatment of an option as an
124
incentive stock option, shares of Common Stock acquired through the exercise of an incentive stock option cannot be disposed of before the later of (i) two years from the date of grant of the option, or (ii) one year from the date of exercise. If the holder satisfies the holding period requirements, the difference between the exercise price and the amount realized upon a subsequent disposition of the shares will constitute long-term capital gain or loss, as the case may be. Assuming both holding periods are satisfied, no deduction will be allowed to us for federal income tax purposes in connection with the grant or exercise of the incentive stock option. If a holder disposes of such shares without meeting the holding period requirements, the participant generally will realize taxable income at the time of such disposition equal to the difference between the exercise price and the lesser of the fair market value of the shares on the date of exercise or the amount realized on the subsequent disposition of the shares, and that amount will generally be deductible by us for federal income tax purposes, subject to the possible limitations on deductibility under Sections 280G and 162(m) of the Code for compensation paid to executives designated in those sections. Finally, if an incentive stock option becomes first exercisable in any one year for shares having an aggregate value in excess of $100,000 (based on the grant date value), the portion of the incentive stock option in respect of those excess shares will be treated as a non-qualified stock option for federal income tax purposes.
No income will be realized by a participant upon grant of a non-qualified option. Upon the exercise of a non-qualified stock option, the participant will recognize ordinary income in an amount equal to the excess, if any, of the fair market value of the underlying exercised shares over the option exercise price paid at the time of exercise. The Company will be able to deduct this same amount for U.S. federal income tax purposes, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Stock Appreciation Rights. No income will be realized by a participant upon grant of a Stock Appreciation Rights (“SAR”). Upon settlement of a SAR, the participant will recognize ordinary income in an amount equal to the fair market value of the payment received in respect of the SAR. The Company will be able to deduct this same amount for U.S. federal income tax purposes, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Restricted Stock. A participant will not be subject to tax at ordinary income rates upon the grant of an award of restricted stock unless the participant otherwise elects to be taxed at the time of grant pursuant to Section 83(b) of the Code. On the date an award of restricted stock becomes transferable or is no longer subject to a substantial risk of forfeiture, the participant will have taxable income equal to the difference between the fair market value of the shares on that date over the amount the participant paid for such shares, if any, unless the participant made an election under Section 83(b) of the Code to be taxed at the time of grant. If the participant made an election under Section 83(b), the participant will have taxable compensation at the time of grant equal to the difference between the fair market value of the shares on the date of grant over the amount the participant paid for such shares, if any. (Special rules apply to the receipt and disposition of restricted shares received by officers and directors who are subject to Section 16(b) of the Exchange Act.) The Company generally will be able to deduct a share-based award, in the same amount and in the same tax year as it is recognized by the participant, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Restricted Stock Units. A participant will not be subject to tax at ordinary income rates upon the grant of a restricted stock unit award. Rather, upon the delivery of shares or cash pursuant to a restricted stock unit award, the participant will have taxable income equal to the fair market value of the number of shares (or the amount of cash) the participant actually receives with respect to the award (less any amount paid by the participant for such restricted stock unit(s)). The Company will be able to deduct the amount of taxable compensation to the participant for U.S. federal income tax purposes, but the deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Other Stock-Based Awards. Generally, participants will recognize taxable income at the time of settlement of other stock-based awards (with the amount of income recognized generally being equal to the fair market value of any shares delivered under the award). The Company will be able to deduct the amount of taxable compensation to the participant for U.S. federal income tax purposes, but the deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those sections.
Tax Deductibility of Compensation Provided Under the 2025 Plan. When a participant recognizes ordinary compensation income as a result of an award granted under the 2025 Plan, the Company may be permitted to claim a federal income tax deduction for such compensation, subject to various limitations that may apply under applicable law (see discussion of Section 162(m) and 280G below, for two examples of limitations that may apply).
125
Section 162(m). Compensation of persons who are “covered employees” is subject to the tax deduction limits of Section 162(m) of the Code. Prior to the enactment of the Tax Cuts and Jobs Act (the “TCJA”) on December 22, 2017, awards that qualified as “performance-based compensation” were exempt from Section 162(m), thereby permitting us to claim the full federal tax deduction otherwise allowed for such compensation. This exception was eliminated by the TCJA. However, pending further guidance, this exception may still be available under state law allowing us to claim the full state deduction otherwise allowed for such compensation. In addition, the TCJA includes a transition rule under which the change described above will not apply to compensation payable pursuant to a written binding contract that was in effect on November 2, 2017 and is not materially modified after that date. To the extent applicable to our existing contracts and awards, our Compensation Committee may avail itself of this transition rule.
Section 280G. To the extent that compensation provided under the 2025 Plan may be deemed to be contingent upon a change in control, a portion of such compensation may be non-deductible by the Company under Section 280G of the Code and may be subject to a 20% excise tax imposed on the recipient of the compensation.
Section 409A. Section 409A of the Code imposes certain restrictions upon the payment of nonqualified deferred compensation. To the extent applicable, it is intended that the 2025 Plan and any grants made thereunder comply with the provisions of Section 409A of the Code, so that the income inclusion provisions of Section 409A(a)(1) of the Code do not apply to the participants. The 2025 Plan and any grants made thereunder will be administered in a manner consistent with this intent.
Securities Available Under the 2025 Plan
Awards
The 2025 Plan allows the Company to make equity and equity-based incentive awards to officers, employees, directors, consultants, and advisors. The Board of Directors anticipates that providing such persons with a direct stake in the Company will assure a closer alignment of the interests of such individuals with those of the Company and its stockholders, thereby stimulating their efforts on the Company’s behalf and strengthening their desire to remain with the Company.
The 2025 Plan provides for the grant of stock options, stock appreciation rights, restricted stock, restricted stock units, and other stock-based awards. All awards will be set forth in an award agreement which will detail all terms and conditions of the awards, including any applicable vesting and payment terms and post-termination exercise limitations.
A brief description of each award type follows.
• Stock Options means an option granted under the 2025 Plan to purchase shares of Common Stock, whether designated as an Incentive Stock Option or a Nonqualified Stock Option;
• Stock Appreciation Rights means a right, designated as a SAR, to receive the appreciation in the fair market value of shares;
• Restricted Stock means shares, subject to a period of restriction or certain other specified restrictions (including, without limitation, a requirement that the participant remain continuously employed or provide continuous service for a specific period of time), granted under the 2025 Plan or issued pursuant to the early exercise of a Stock Option;
• Restricted Stock Units means an unfunded and unsecured promise to deliver shares, cash, other securities, or other property, subject to certain restrictions (including, without limitation, a requirement that the participant remain continuously employed or provide continuous service for a specific period of time) granted under the 2025 Plan; and
• Other-Stock Based Awards means any other awards not specifically described in the 2025 Plan that are valued in whole or in part by reference to, or are otherwise based on, shares and are created by the plan administrator pursuant to the 2025 Plan.
126
The maximum aggregate number of shares that may be issued under the 2025 Plan is ten percent (10%) of the shares outstanding on [—], 2025 (the “Plan Share Limit”). The shares subject to the 2025 Plan may be authorized, but unissued, or reacquired shares. On the first day of each calendar year during the term of the 2025 Plan, commencing on January 1, 2026 and continuing until (and including) January 1, 2035, the number of shares available under the Plan Share Limit shall automatically increase by a number equal to the lesser of (i) three percent (3%) of the total number of shares issued and outstanding on December 31 of the calendar year immediately preceding of the date of such increase and (ii) a number of shares determined by the Board of Directors.
Upon payment in shares pursuant to the exercise or settlement of an award, the number of shares available for issuance under the 2025 Plan shall be reduced only by the number of shares actually issued in such payment. If a participant pays the exercise price (or purchase price, if applicable) of an award through the tender of shares, or if the shares are tendered or withheld to satisfy any tax withholding obligations, the number of shares so tendered or withheld shall again be available for issuance pursuant to future awards under the 2025 Plan, although such shares shall not again become available for issuance as incentive stock options. Shares shall not be deemed to have been issued pursuant to the 2025 Plan with respect to any portion of an award that is settled in cash. If any outstanding award expires or is terminated or canceled without having been exercised or settled in full, or if the shares acquired pursuant to an award subject to forfeiture or repurchase are forfeited or repurchased by the Company, the shares allocable to the terminated portion of such award or such forfeited or repurchased shares shall again be available for grant under the 2025 Plan. No more than ten percent (10%) of the shares outstanding on [—], 2025 (subject to adjustment pursuant to the 2025 Plan) may be issued under the 2025 Plan upon the exercise of incentive stock options.
The 2025 Plan will be administered by a committee of at least one person as the Board of Directors may appoint or, if no such committee has been appointed by the Board of Directors, the 2025 Plan shall be administered by the Board of Directors (the “Administration Committee”). The plan administrator, which initially will be the Administration Committee, has full power to select, from among the individuals eligible for awards, the individuals to whom awards will be granted, to make any combination of awards to participants, and to determine the specific terms and conditions of each award, subject to the provisions of the 2025 Plan. The plan administrator may delegate to one or more officers of the Company some or all of its authority under the 2025 Plan, including the authority to grant all types of awards to individuals who are not subject to the reporting and other provisions of Section 16 of the Exchange Act, subject to certain limitations and guidelines.
Persons eligible to participate in the 2025 Plan will be officers, employees, non-employee directors, consultants, and advisors of the Company and its subsidiaries as selected from time to time by the plan administrator in its discretion. As of the date of this Information Statement, approximately [—] individuals are eligible to participate in the 2025 Plan, which includes approximately [—] officers, no employees who are not officers, [—] non-employee directors, and [—] consultants/independent contractors.
Stock Options
The 2025 Plan permits the granting of stock options to officers, employees, non-employee directors, consultants, and advisors of the Company and its subsidiaries as selected from time to time by the plan administrator in its discretion. The per share exercise price for shares to be issued pursuant to exercise of a stock option will by determined by the plan administrator but shall be no less than 100% of the fair market value per share on the date of grant.
The exercise period of stock options shall be determined by the plan administrator, subject to the limitation set forth in the 2025 Plan. Upon exercise of the stock options, the exercise price must be paid by either (i) cash, (ii) check, (iii) if approved by the plan administrator, as determined in its sole discretion, surrender of other shares which meet the conditions established by the plan administrator to avoid adverse accounting consequences to the Company (as determined by the plan administrator), (iv) if approved by the plan administrator, as determined in its sole discretion, by a broker-assisted cashless exercise in accordance with procedures approved by the plan administrator, (v) if approved by the plan administrator for a nonqualified stock option, as determined in its sole discretion, by delivery of a notice of “net exercise” to the Company, pursuant to which the participant shall receive the number of shares underlying the stock option so exercised reduced by the number of shares equal to the aggregate exercise price of the stock option divided by the fair market value on the date of exercise, and (vi) such other method of payment permitted by applicable law.
127
Stock Appreciation Rights
The plan administrator may award stock appreciation rights subject to such conditions and restrictions as it may determine. Stock appreciation rights entitle the recipient to shares of common stock of the Company, or cash, equal to the value of the appreciation in the Company’s stock price over the exercise price. The exercise price generally may not be less than 100% of the fair market value of common stock of the Company on the date of grant. The plan administrator will determine at what time or times each stock appreciation right may be exercised, including the ability to accelerate the vesting of such stock appreciation rights.
Restricted Stock and Restricted Stock Units
The plan administrator may award restricted shares of common stock of the Company and restricted stock units to participants subject to such conditions and restrictions as it may determine. These conditions and restrictions may include the achievement of certain performance goals and/or continued employment with the Company through a specified vesting period. Unless the plan administrator determines otherwise, restricted stock will be held by the Company as escrow agent until the restrictions on such restricted stock have lapsed. The plan administrator, in its discretion, may accelerate the time at which any restrictions will lapse or be removed. Restricted stock may be granted to participants in recognition of past services or for other valid consideration and may be issued in lieu of cash compensation due to such participant. The plan administrator may grant dividend equivalent rights to participants that entitle the recipient to receive credits for dividends that would be paid if the recipient had held a specified number of shares of common stock of the Company.
Other Stock-Based Awards
Other stock-based awards may be granted either alone, in addition to, or in tandem with, other awards granted under the 2025 Plan and/or cash awards made outside of the 2025 Plan. The plan administrator shall have authority to determine the participants to whom and the time or times at which other stock-based awards shall be made, the amount of such other stock-based awards, and all other conditions of the other stock-based awards including any dividend and/or voting rights.
Other Material Features of the 2025 Plan
The 2025 Plan requires the plan administrator to make appropriate adjustments to the number of shares of common stock that are subject to the 2025 Plan, to certain limits in the 2025 Plan, and to any outstanding awards to reflect stock dividends, stock splits, extraordinary cash dividends and similar events.
In the event of a change in control, each outstanding award shall be assumed or an equivalent award substituted by the acquiring or successor corporation or a parent of the acquiring or successor corporation. Unless determined otherwise by the plan administrator, in the event that the successor corporation refuses to assume or substitute the award, (A) the participant shall fully vest in and have the right to exercise the award as to all of the shares, including those as to which it would not otherwise be vested or exercisable; (B) all applicable restrictions will lapse; and (C) all performance objectives and other vesting criteria will be deemed achieved at targeted levels. If a stock option or a stock appreciation right is not assumed or substituted in the event of a change in control, the plan administrator shall notify the respective participant that the stock option or the stock appreciation right shall be exercisable, to the extent vested, for a period of up to fifteen (15) days from the date of such notice, and the stock option or stock appreciation right shall terminate upon the expiration of such period.
Unless determined otherwise by the plan administrator, an award may not be sold, pledged, assigned, hypothecated, transferred, or disposed of in any manner, except to the participant’s estate or legal representative, and may be exercised, during the lifetime of the participant, only by the participant, although the plan administrator, in its discretion, may permit award transfers for purposes of estate planning or charitable giving.
The Board of Directors may at any time amend, alter, suspend, or terminate the 2025 Plan. The Company may obtain stockholder approval of the 2025 Plan to the extent necessary or, as determined by the plan administrator, desirable to comply with applicable laws, including any amendment that (i) increases the number of shares available for issuance under the 2025 Plan, or (ii) changes the persons or class of persons eligible to receive awards.
No awards under the 2025 Plan have been made prior to the date of this Information Statement.
128
NYSE American Rules and the Necessity for Stockholder Approval
As described above, we are subject to NYSE American rules, including NYSE American Company Guide 711, pursuant to which approval of stockholders is required with respect to the establishment of (or material amendment to) a stock option or purchase plan or other equity compensation arrangement pursuant to which options or stock may be acquired by officers, directors, employees, or consultants, regardless of whether or not such authorization is required by law or by the company’s charter. Therefore, the Consenting Stockholders approved the 2025 Plan.
Stockholder Approval of the 2025 Plan
The approval of the 2025 Plan, including for purposes of NYSE American rules, required the affirmative approval of a majority of the holders of the outstanding shares of the Company’s Common Stock that were entitled to approve the 2025 Plan.
As of the Record Date, the Company had [—] shares of Common Stock issued and outstanding and the Consenting Stockholder held [—] shares of Common Stock as of the Record Date, representing approximately [—]% of the voting power of all shares of the Company’s Common Stock. The Consenting Stockholders approved the 2025 Plan upon execution of the Written Consent on [—], 2025, following execution of the Merger Agreement.
This Information Statement is first being mailed on or about [—], 2026, to the Company’s stockholders of record as of the Record Date. The 2025 Plan shall be effective on or about [—], 2026, or approximately 20 days after we mail this Information Statement.
129
130
MANAGEMENT OF APIMEDS PHARMACEUTICALS US, INC.
The following table sets forth information regarding the Company’s executive officers and non-employee directors.
|
Name |
Age |
Position |
||
|
Dr. Vin Menon |
[—] |
Chief Executive Officer |
||
|
Erick Frim |
[—] |
Chief Financial Officer |
||
|
Christopher Kim, MD. |
[—] |
Chief Medical Officer |
||
|
Jakap Koo |
[65] |
Director |
||
|
Dr. Bennett Weintraub, PhD. |
[57] |
Director |
||
|
Carol O’Donnell |
[68] |
Director |
||
|
Elona Kogan |
[56] |
Director |
Dr. Vin Menon — Chief Executive Officer
Dr. Vin Menon has been our Chief Executive Officer since December 2025. Dr. Menon is a veteran in the technology services industry, who can be credited with the strategic direction behind several disruptive technology companies. In the corporate world, he has held various leadership positions at multinational corporations like HP & Compaq with global responsibilities. Driven by his passion for technology and innovation, Dr. Menon has been a forerunner in technological innovation and has helped create the business ecosystem of disruptive technologies and high-growth companies. His experience has helped him in the technology space as an entrepreneur and advisor, leading several startups from inception to meteoric growth across continents. Dr. Menon’s proven track record of setting up motivated and high caliber teams in the technology and services industry, establishing development centers from scratch to scale, and building company competencies led him to being awarded ‘Entrepreneur of the Year 2012’ by Rotary-ASME, ‘Outstanding Entrepreneur Award 2011’ by APEA, the ‘Spirit of Enterprise 2010’ by SOE Singapore. Dr. Menon was also selected as a ‘Leading Indian Entrepreneur of the Year 2010’ by the Singapore Indian Chamber of Commerce. From the years 2021 to 2023 he co-founded and served as Strategic Advisor to CGCX, a Fintech, decentralized finance, and digital assets platform, where he provided strategic advisory services and growth initiatives. He currently serves as Chief Executive Officer of AQUAE Impact (AQUAE Impact Exchange Co. L.L.C/AQUAE Impact), a sustainable financial and environmental assets platform that uses blockchain technology and artificial intelligence, which he co-founded and currently forms part of its executive leadership providing oversight of product and sustainability initiatives. He also currently serves as Chief Executive Officer of AQUAE Labs Pte Ltd, which is the research and development and technology arm of AQUAE Impact, where he provides product and technology leadership, measurement, reporting, and verification of environmental credits. Additionally, he currently occupies the role of Strategic Advisor of TechyTrade FZ-LLC, which is a bitcoin-backed company that operates in the digital asset and treasury innovation space. Dr. Menon is also a champion of techno-preneurship and was serving on the Board of Directors of the Spirit of Enterprise (SOE) and the Mentoring Programme under Action Community for Entrepreneurship (ACE) by SPRING Singapore. Moreover, he completed his bachelor’s degree in computer applications from India, with first-class honors. He has also completed the following programs: Advanced Management Program (AMP) at NTU-Berkeley (Haas Business School, California) and Advanced Management Program (AMP) at The Wharton School (University of Pennsylvania, USA) specialized in Finance. Dr. Menon obtained an EMBA from the Nanyang Technological University (NTU) in Singapore. Lastly, he completed his PhD, Blockchain for Impact in Healthcare from The Open International University for Complementary Medicine in collaboration with Al-Farabi Kazakh National University, Kazakhstan 2019. We believe that these experiences provide Dr. Menon with the skills necessary to lead the Company as its Chief Executive officer, including overseeing the Company’s strategy, operations, financial performance, and overall corporate governance.
Erick Frim — Chief Financial Officer
Erick Frim has over 40 years of experience as an accountant, financial executive and consultant. Mr. Frim joined Apimeds as CFO in July of 2025. In 2019, he joined CFO Squad and as a partner in the CFO Squad, LLC Mr. Frim advised clients on technical accounting and regulatory compliance, assisting numerous companies with their initial public offerings. Prior to the CFO Squad, Mr. Frim served as a director in the public company audit practice of EisnerAmper LLP. Mr. Frim as also served as a financial executive for digital media pioneer DIVA Systems Corporation. A former CPA, he has a BS in Accounting from Ball State University.
131
Christopher Kim, MD. — Chief Medical Officer
Dr. Christopher Kim has been our Chairman and Chief Medical Officer since our inception and served as our interim Chief Executive Officer from July 2022 to September 2023. Dr. Kim is the inventor and developer of Apitox and the founder of Apimeds Korea, where he has served as a director since its inception. Mr. Kim served as the Chief Executive Officer of Apimeds Korea from May 2003 to August 2011. Prior to founding Apimeds Korea, Dr. Kim lead with the support of Guju Pharmaceuticals, clinical trials for Apitoxin in Korea, which was approved by the Korea Food and Drug Administration in 2003 for relief of pain and inflammation for patients with Osteoarthritis. In 2005, he began focusing on the clinical development of Apitox in the United States, including the first of two-Phase III clinical studies for Osteoarthritis. Prior to his time with Apimeds Korea, Dr. Kim served as the President of the International Pain Institute of New Jersey from January 1983 to May 2003, a center for chronic pain and other disabling diseases that conducted clinical research and provided treatment. He served as a professor at Biomedical Center, CHA Graduate School of Medicine in Korea from March 2005 to February 2017. Dr. Kim is a licensed physician in New Jersey, New York and Korea and a Pain Medicine Specialist (American Board). Over the past twenty years, Dr. Kim has treated thousands of chronically disabled patients with autoimmune diseases, including MS. Dr. Kim received his medical degree from the School of Medicine, CN University in Korea.
We believe Dr. Kim’s extensive experience in pharmaceutical development and the biopharmaceutical industry, as well as his research and treatment of autoimmune diseases, and institutional knowledge of our product candidate, qualifies him to serve as of Chief Medical Officer.
Jakap Koo — Director
Mr. Jakap Koo has served as a director since October 2023. Mr. Koo is also the Chief Executive Officer and President of both Apimeds Korea and its parent company, Inscobee Inc. (KRX: 006490), where he has served since March 2020. Before joining Apimeds Korea and Inscobee, from March 2015 to December 2019 he served as the Chief Executive Officer at Lotte Auto Lease Co. Ltd., where he grew company revenue through various financial services of car rental, installment payment, automobile leasing and investment banking to both B2B and B2C clients. Mr. Koo has spent more than 35 years mostly as C-level executives in various financial institutions and IT companies. His management and operational experiences cover banking, asset management, venture capital, private equity, and biotechnology companies. Mr. Koo has received his MBA from Stern School of New York University. He graduated from Seoul National University majoring in Law.
We believe Mr. Koo’s extensive financial knowledge qualifies him to serve on our Board of Directors.
Independent Directors:
Dr. Bennett Weintraub, PhD.
Dr. Weintraub has served as a director since October 2023. Dr. Weintraub currently serves as the President of inThought Research (“inThought”), a healthcare business intelligence consulting firm which he founded in 2009. inThought provides business development support, competitive intelligence monitoring, medical conference coverage, and other services both to professional investors and to pharma/biotech companies. Dr. Weintraub has also served as the Chief Scientific Officer of inPhronesis since 2018.
After completing his training in immunology and biochemistry, Dr. Weintraub co-founded Biotech Tracker, an online tool for investors, where he served as a financial analyst from 2000 to 2008. From 2006 to 2008, Dr. Weintraub served as an analyst at Reuters Insight, providing analysis of drug development and trends in medicine to professional investors. Dr. Weintraub served as a licensed security analyst with Variant Research from 2005 to 2006.
From 1999 to 2000, Dr. Weintraub was senior scientific editor for the biology research journals Cell and Molecular Cell. Dr. Weintraub performed biochemistry and immunology research at Stanford University and at the John Curtin School of Medical Research in Canberra, Australia. He earned his doctorate in Biology from the University of California, San Diego, and a Bachelor of Science in Life Science from the Massachusetts Institute of Technology.
We believe Mr. Weintraub’s extensive science background qualifies him to serve on our Board of Directors.
132
Carol O’Donnell
Carol O’Donnell has served as a director since October 2023. Ms. O’Donnell is currently a Director and Member of the Audit Committee of Sono-Tek Corporation (NASDAQ: SOTK), where she has served since November 2018. Prior to that, she served as General Counsel to Boothbay Fund Management LLC, a registered investment adviser, from December 2019 through May 2021. Ms. O’Donnell joined Protégé Partners and MOV37, an industry leading firm investing in and seeding smaller and emerging hedge fund managers in April 2016 and has served as Chief Executive Officer since January 2018. Prior to joining Protégé Partners and MOV37, Ms. O’Donnell was the Director of Legal and Compliance with DARA Capital US, Inc., a Swiss-owned boutique registered investment advisory and wealth management firm from January 2013 to March 2016. She served as General Counsel and Chief Compliance Officer of each of the Permal Group and Framework Investment Group from June 2004 through February 2011 and from January 2002 to May 2004, respectively. She also served as a director of FSI Low Beta from 2012 to 2021. Ms. O’Donnell was named one of the Top 50 Women in Hedge Funds in September 2018 and is currently admitted to practice law in the State of Connecticut.
We believe Ms. O’Donnell’s extensive experience in the financial industry qualifies her to serve on our Board of Directors.
Elona Kogan
Elona Kogan has served as a director since October 2024. Beginning in August 2024, Most recently, Ms. Kogan served as the Chief Legal Officer of Terns Pharmaceutical, Inc. (Nasdaq: TERNS), a publicly traded biopharmaceutical company, Prior to joining us, from November 2020 through August 2024, Ms. Kogan served as the General Counsel and Chief Legal Officer of Seer Inc. (Nasdaq: SEER), a publicly traded life science company. From May 2018 through August 2021, Ms. Kogan served as a director of Cardax, Inc., a biotechnology company operating in the wellness health care space. From March 2019 through August 2020, Ms. Kogan served as the General Counsel of Selecta Biosciences, Inc., a clinical-stage biotechnology company. Ms. Kogan is a graduate of Southwestern University School of Law. Ms. Kogan graduated from Columbia University, Barnard College, with a B.A. in Economics.
We believe Ms. Kogan’s extensive experience as an operating executive in biopharmaceutical and life science space, in addition to her experience leading government relations, and serving as general counsel and chief legal officer of other publicly traded companies qualifies her to serve on our Board of Directors.
133
BENEFICIAL OWNERSHIP OF SECURITIES
The following table shows information, as of [January 9], 2026, concerning the beneficial ownership of our Common Stock by: (i) each person we know to be the beneficial owner of more than five percent (5%) of our Common Stock; (ii) each of our current directors; (iii) each of our named executive officers; and (iv) all current directors and executive officers as a group. Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she, or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days.
The beneficial ownership of our Common Stock is based on [12,575,983] shares of our Common Stock issued and outstanding as of [January 9], 2026.
Unless otherwise indicated in the footnotes to the table below, and subject to applicable community property laws, the Company believes that all persons named in the table below have sole voting and investment power with respect to their beneficially owned shares of Common Stock.
|
Name of Beneficial Owner |
Number of |
% of |
|||
|
Directors and Named Executive Officers: |
|
||||
|
Dr. Vin Menon |
— |
— |
|
||
|
Erick Frim |
— |
— |
|
||
|
Jakap Koo |
615,385 |
4.893 |
% |
||
|
Christopher Kim, MD. |
— |
— |
|
||
|
Bennett Weintraub, PhD |
— |
— |
|
||
|
Elona Kogan |
— |
— |
|
||
|
Carol O’Donnell |
— |
— |
|
||
|
All directors and officers as a group (7 individuals) |
615,385 |
4.893 |
% |
||
|
5% or Greater Stockholders: |
|
||||
|
Apimeds Inc.(1) |
4,316,618 |
34.324 |
% |
||
|
Inscobee Inc.(2) |
1,599,747 |
12.721 |
|
||
|
Dominus IB, Inc(3) |
800,000 |
6.361 |
% |
||
____________
(1) Apimeds Inc. is a wholly owned subsidiary of Inscobee Inc. Inscobee Inc. has voting and investment control over the shares held by Apimeds Inc. Millenium Holdings is controlled by You In Soo, and as such, Mr. Yoo may be deemed to have beneficial ownership over the shares held by both Inscobee Inc. and Apimeds Inc. The business address for Apimeds Inc. is [—]. The business address for Millenium Holdings is 107, Gasan Digital 2-ro, Geumcheon-gu, Seoul, Korea. Each of the parties named in this footnote disclaims any beneficial ownership of the reported shares other than to the extent of any pecuniary interest the party may have therein.
(2) Represents shares held directly by Inscobee Inc. Millenium Holdings has voting and investment control with respect to the shares held by Inscobee Inc. Millenium Holdings is controlled by You In Soo, and as such, Mr. Yoo may be deemed to have beneficial ownership over the shares held by both Inscobee Inc. and Apimeds Inc. The business address for Inscobee Inc. is Room 613, Digital-ro 130, 6F, Geumcheon-gu, Seoul, 08580 Republic of Korea. The business address for Millenium Holdings is 107, Gasan Digital 2-ro, Geumcheon-gu, Seoul, Korea. Each of the parties named in this footnote disclaims any beneficial ownership of the reported shares other than to the extent of any pecuniary interest the party may have therein.
(3) Dominus IB, Inc. is controlled by its Chief Executive Officer and largest shareholder, Park Kyoung Jin, who may be deemed to have voting and investment control with respect to the shares held by Dominus IB, Inc. The business address for Dominus IB, Inc. is 144, Dobong-ro, Gangbuk, Seoul, Republic of Korea.
134
The following summary of the capital stock of the Company does not purport to be complete and is qualified in its entirety by reference to our Charter and our amended and restated bylaws (the “Bylaws”). Unless the context requires otherwise, all references to the “Company,” “Apimeds,” “we,” “our,” and “us” in this Exhibit refer to Apimeds Pharmaceuticals US, Inc.
General
The following descriptions of our capital stock and certain provisions of our Charter and Bylaws are summaries. The full text of our Charter and our Bylaws are filed as Annexes K and L, respectively. We urge you to read our Charter and our Bylaws in their entirety for a complete description of the rights and preferences of our capital stock.
Authorized Capitalization
Our Charter authorizes the issuance of 100,000,000 shares of common stock, par value $0.01 per share and 10,000,000 shares of preferred stock, par value $0.01 per share.
Forward Stock Split
On January 6, 2022, we amended our Charter to effect a forward stock split of our outstanding shares of common stock by a ratio of 1-for-10,000.
Reverse Stock Split
On February 25, 2025, we amended our Charter to effect a 1-for-2.6 reverse stock split, pursuant to which each 2.6 shares of common stock held of record by the holder thereof were reclassified into one share of common stock. No fractional shares were issued.
As of the date of this Information Statement, there were [—] shares of common stock issued and outstanding held of record by [—] stockholders and [—] shares of preferred stock issued and outstanding.
Upon completion of the Preferred Stock Conversion, the Notes Conversion, and the Reverse Stock Split, there will be [—] shares of common stock outstanding.
Common Stock
Voting Rights. The holders of shares of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders.
Dividends. The board of directors of our company may cause dividends to be paid to the holders of shares of common stock out of funds legally available for the payment of dividends by declaring an amount per share as a dividend. When and as dividends are declared on the common stock, whether payable in cash, in property or in shares of stock or other securities of our company, the holders of common stock shall be entitled to share ratably according to the number of shares of common stock held by them, in such dividends.
Liquidation Rights. In the event of any voluntary or involuntary liquidation, dissolution or winding up of the affairs of our company, the holders of shares of common stock shall be entitled to share ratably, according to the number of shares of common stock held by them, in all remaining assets of our company available for distribution to its stockholders.
Blank Check Preferred Stock
Our board of directors has the authority to issue undesignated shares of “blank check” preferred stock in one or more series and to fix the designation, relative powers, preferences and rights and qualifications, limitations or restrictions of all shares of each such series, including, without limitation, dividend rates, conversion rights, voting rights, redemption and sinking fund provisions, liquidation preferences and the number of shares constituting each such series, without any further vote or action by the stockholders. The issuance of additional preferred stock could decrease the amount of
135
earnings and assets available for distribution to holders of our common stock or adversely affect the rights and powers, including voting rights, of the holders of our common stock and could, among other things, have the effect of delaying, deferring or preventing a change in control of our company without further action by the stockholders. We have no present plans to issue any shares of preferred stock.
Anti-Takeover Provisions of the Charter and Bylaws
Stockholder Action; Special Meetings of Stockholders
The Bylaws provide that stockholders may not take action by written consent, but may only take action at annual or special meetings of stockholders. As a result, a holder controlling a majority of Company capital stock would not be able to amend the Bylaws or remove directors without holding a meeting of stockholders called in accordance with the Bylaws. Further, the Bylaws provide that only the chairperson of the Board, a majority of the board of directors or the Chief Executive Officer may call special meetings of stockholders, thus prohibiting a stockholder from calling a special meeting. These provisions might delay the ability of stockholders to force consideration of a proposal or for stockholders controlling a majority of Company capital stock to take any action, including the removal of directors.
Advance Notice Requirements for Stockholder Proposals and Director Nominations
In addition, the Bylaws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting or special meeting of stockholders. Generally, in order for any matter to be “properly brought” before a meeting, the matter must be (a) specified in a notice of meeting given by or at the direction of the board of directors, (b) if not specified in a notice of meeting, otherwise brought before the meeting by the board of directors or the chairperson of the meeting, or (c) otherwise properly brought before the meeting by a stockholder present in person who (1) was a stockholder at the time of giving the notice, (2) is entitled to vote at the meeting, and (3) has complied with the advance notice procedures specified in the Bylaws or properly made such proposal in accordance with Rule 14a-8 under the Exchange Act and the rules and regulations thereunder, which proposal has been included in the proxy statement for the annual meeting. Further, for business to be properly brought before an annual meeting by a stockholder, the stockholder must (a) provide Timely Notice (as defined below) thereof in writing and in proper form to the secretary and (b) provide any updates or supplements to such notice at the times and in the forms required by the Bylaws. To be timely, a stockholder’s notice must be delivered to, or mailed and received at, the Company’s principal executive offices not less than ninety (90) days nor more than one hundred twenty (120) days prior to the one-year anniversary of the preceding year’s annual meeting; provided, however, that if the date of the annual meeting is more than thirty (30) days before or more than thirty (30) days after such anniversary date, notice by the stockholder to be timely must be so delivered, or mailed and received, not earlier than the close of business on the 120th day prior to such annual meeting and not later than the 90th day prior to such annual meeting or, the 10th day following the day on which public disclosure of the date of such annual meeting was first made (such notice within such time periods, “Timely Notice”). These provisions could have the effect of delaying stockholder actions that are favored by the holders of a majority of the outstanding voting securities until the next stockholder meeting.
No Cumulative Voting
Under the DGCL, there is no right to vote cumulatively (which allows stockholders to cast all of the votes such stockholder is entitled to for a single nominee for a board of directors rather than only being able to vote the number of shares such stockholder holds for or against each nominee) unless expressly authorized in the certificate of incorporation. The Charter does not authorize cumulative voting.
Amendment of Charter or Bylaws
The DGCL provides generally that the affirmative vote of a majority of the outstanding stock entitled to vote on amendments to a corporation’s certificate of incorporation or bylaws is required to approve such amendment, unless a corporation’s certificate of incorporation or bylaws, as the case may be, requires a greater percentage. Our Bylaws may be amended or repealed by a majority vote of the board of directors or by the holders of at least 66 2/3% of the voting power of all of the then-outstanding shares entitled to vote generally in the election of directors, voting together as a single class.
136
Authorized but Unissued Capital Stock
Delaware law does not require stockholder approval for any issuance of authorized shares. However, the listing requirements of the NYSE American, which would apply if and so long as the common stock remains listed on the NYSE American, require stockholder approval of certain issuances equal to or exceeding 20% of the then outstanding voting power or then outstanding number of shares of common stock. Additional shares that may be issued in the future may be used for a variety of corporate purposes, including future public offerings, to raise additional capital or to facilitate acquisitions.
One of the effects of the existence of unissued and unreserved common stock may be to enable our company to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of our company by means of a merger, tender offer, proxy contest or otherwise and thereby protect the continuity of management and possibly deprive stockholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. We have entered into, or expect to enter into, agreements to indemnify our directors, executive officers and other employees as determined by our board of directors.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, stockholders will have appraisal rights in connection with a merger or consolidation of the Company. Pursuant to Section 262 of the DGCL, stockholders who properly demand and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of the Company’s stockholders may bring an action in the company’s name to procure a judgment in its favor, also known as a derivative action, provided that the stockholder bringing the action is a holder of the Company’s shares at the time of the transaction to which the action relates.
Forum Selection
The Bylaws provide that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for the following types of actions or proceedings under Delaware statutory or common law: (i) any derivative action or proceeding brought on behalf of the Company; (ii) any action or proceeding asserting a claim of breach of a fiduciary duty owed by any of the Company’s current or former directors, officers, or other of the Company or its stockholders; (iii) any action or proceeding asserting a claim against the Company or any of its current or former directors, officers, or other employees, arising out of or pursuant to any provision of the Delaware General Corporation Law, the Charter or the Bylaws; (iv) any action or proceeding to interpret, apply, enforce, or determine the validity of the Charter or the Bylaws; (v) any action or proceeding as to which the DGCL confers jurisdiction to the Court of Chancery of the State of Delaware; and (vi) any action asserting a claim against the Company or any of its directors, officers, or other employees governed by the internal affairs doctrine, in all cases to the fullest extent permitted by law and subject to the court’s having personal jurisdiction over the indispensable parties named as defendants. These provisions would not apply to suits brought to enforce a duty or liability created by the Exchange Act. Furthermore, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all such Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. To prevent having to litigate claims in multiple jurisdictions and the threat of inconsistent or contrary rulings by different courts, among other considerations, the Bylaws further provide that the federal district courts of the United States of America will be the exclusive forum for resolving any complaint asserting a cause or causes of action arising under the Securities Act, including all causes of action asserted against any defendant to such complaint. For the avoidance of doubt, this provision is intended to
137
benefit and may be enforced by the Company, its officers and directors, the underwriters to any offering giving rise to such complaint, and any other professional entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying this offering. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions. In such instance, the Company would expect to vigorously assert the validity and enforceability of the exclusive forum provisions of the Bylaws.
These exclusive forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with the Company or its directors, officers, or other employees and may discourage these types of lawsuits. Furthermore, the enforceability of similar choice of forum provisions in other companies’ certificates of incorporation or bylaws has been challenged in legal proceedings, and it is possible that a court could find these types of provisions to be inapplicable or unenforceable. We note that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock upon the closing of this offering will be VStock Transfer, LLC. The transfer agent and registrar’s address are 18 Lafayette Place, Woodmere, NY 11598.
National Securities Exchange Listing
We have received approval to have our common stock listed on the NYSE American under the symbol “APUS.”
138
DELIVERY OF DOCUMENTS TO SECURITY HOLDERS SHARING AN ADDRESS
Regulations regarding the delivery of copies of information statements to stockholders permit us, banks, brokerage firms and other nominees to send one information statement to multiple stockholders who share the same address under certain circumstances. This practice is known as “householding.” Stockholders who hold their shares through a bank, broker or other nominee may have consented to reducing the number of copies of materials delivered to their address. In the event that a stockholder wishes to revoke a “householding” consent previously provided to a bank, broker or other nominee, the stockholder must contact the bank, broker or other nominee, as applicable, to revoke such consent. If a stockholder wishes to receive a separate information statement, we will promptly deliver a separate copy to such stockholder that contacts us at 100 Matawan Rd, Suite 325 Matawan, New Jersey 07747; or by telephone at: [—]. Any stockholders of record sharing an address who now receive multiple copies of our annual reports, proxy statements and information statements, and who wish to receive only one copy of these materials per household in the future should also contact the Company’s secretary by mail or telephone as instructed above. Any stockholders sharing an address whose shares of our common stock are held by a bank, broker or other nominee who now receive multiple copies of our annual reports, proxy statements and information statements, and who wish to receive only one copy of these materials per household, should contact the bank, broker or other nominee to request that only one set of these materials be delivered in the future.
WHERE YOU CAN FIND MORE INFORMATION
The Company files annual, quarterly, and current reports, proxy statements, and other information with the SEC. The Company’s filings with the SEC are available free of charge on the SEC’s EDGAR system at http://www.sec.gov.
PLEASE NOTE THAT THIS IS NOT A REQUEST FOR YOUR VOTE OR A PROXY STATEMENT, BUT RATHER AN INFORMATION STATEMENT DESIGNED TO INFORM YOU OF CERTAIN TRANSACTIONS ENTERED INTO BY THE COMPANY.
WE ARE NOT ASKING YOU FOR A PROXY AND YOU ARE REQUESTED NOT TO SEND US A PROXY.
|
By Order of the Board of Directors of Apimeds Pharmaceuticals US, Inc. |
||
|
/s/ |
||
|
Vin Menon |
139
APIMEDS PHARMACEUTICALS US, INC. Unaudited FINANCIAL STATEMENTS
APIMEDS PHARMACEUTICALS US, INC. audited FINANCIAL STATEMENTS
|
Page |
||
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM (PCAOB ID: 6651) |
F-21 |
|
|
Financial Statements |
||
|
F-22 |
||
|
Statements of Operations for the Years Ended December 31, 2024 and 2023 |
F-23 |
|
|
F-24 |
||
|
Statements of Cash Flows for the Years Ended December 31, 2024 and 2023 |
F-25 |
|
|
F-26 |
MINDWAVE INNOVATIONS INC. Unaudited FINANCIAL STATEMENTS
UNAUDITED CONSOLIDATED FINANCIAL STATEMENTS AS OF SEPTEMBER 30, 2025 AND FOR THE SIX MONTHS ENDED SEPTEMBER 30, 2025 AND 2024
|
Page |
||
|
F-37 |
||
|
F-38 |
||
|
F-39 |
||
|
F-40 |
||
|
F-41 |
MINDWAVE INNOVATIONS INC. audited FINANCIAL STATEMENTS
|
Page |
||
|
F-52 |
||
|
CONSOLIDATED FINANCIAL STATEMENTS |
||
|
F-53 |
||
|
F-54 |
||
|
F-55 |
||
|
F-56 |
||
|
F-57 |
F-1
Apimeds Pharmaceuticals US, Inc.
Unaudited Condensed Balance Sheets
|
September 30, |
December 31, |
|||||||
|
(unaudited) |
||||||||
|
Assets |
|
|
|
|
||||
|
Current assets: |
|
|
|
|
||||
|
Cash |
$ |
6,986,617 |
|
$ |
3,455 |
|
||
|
Prepaid expenses and other current assets |
|
2,099,491 |
|
|
9,602 |
|
||
|
Total current assets |
|
9,086,108 |
|
|
13,057 |
|
||
|
|
|
|
|
|||||
|
Property and equipment, net |
|
34,188 |
|
|
— |
|
||
|
Long-term portion of prepaid expenses |
|
129,740 |
|
|
— |
|
||
|
Total assets |
$ |
9,250,036 |
|
$ |
13,057 |
|
||
|
|
|
|
|
|||||
|
Liabilities and shareholders’ equity |
|
|
|
|
||||
|
Current liabilities: |
|
|
|
|
||||
|
Accounts payable and accrued expenses |
$ |
567,633 |
|
$ |
591,191 |
|
||
|
Accrued interest – related party |
|
21,651 |
|
|
106,643 |
|
||
|
Advance payable to related party |
|
100 |
|
|
76,500 |
|
||
|
Notes payable – related party |
|
500,000 |
|
|
250,000 |
|
||
|
Total current liabilities |
|
1,089,384 |
|
|
1,024,334 |
|
||
|
|
|
|
|
|||||
|
Long-term liabilities |
|
|
|
|
||||
|
Long-term convertible notes payable – related party |
|
— |
|
|
346,844 |
|
||
|
Total liabilities |
|
1,089,384 |
|
|
1,371,178 |
|
||
|
|
|
|
|
|||||
|
Commitments and contingencies (note 8) |
|
— |
|
|
— |
|
||
|
|
|
|
|
|||||
|
Shareholders’ equity: |
|
|
|
|
||||
|
Preferred stock, par value $0.01, 10,000,000 shares authorized; none issued and outstanding as of September 30, 2025 and December 31, 2024 |
|
— |
|
|
— |
|
||
|
Common stock, par value $0.01, 100,000,000 shares authorized; 12,575,983 and 7,903,850 issued and outstanding as of September 30, 2025 and December 31, 2024, respectively |
|
125,760 |
|
|
79,039 |
|
||
|
Additional paid-in capital |
|
17,272,661 |
|
|
2,954,764 |
|
||
|
Accumulated deficit |
|
(9,237,769 |
) |
|
(4,391,924 |
) |
||
|
Total shareholders’ equity (deficit) |
|
8,160,652 |
|
|
(1,358,121 |
) |
||
|
|
|
|
|
|||||
|
Total liabilities and shareholders’ equity |
$ |
9,250,036 |
|
$ |
13,057 |
|
||
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-2
Apimeds Pharmaceuticals US, Inc.
Unaudited Condensed Statements of Operations
|
For the three months ended |
For the nine months ended |
|||||||||||||||
|
2025 |
2024 |
2025 |
2024 |
|||||||||||||
|
Operating expenses: |
|
|
|
|
|
|
|
|
||||||||
|
Research and development expenses |
$ |
619,693 |
|
$ |
— |
|
$ |
1,271,477 |
|
$ |
— |
|
||||
|
General and administrative expenses |
|
1,224,546 |
|
|
299,999 |
|
|
3,601,034 |
|
|
999,482 |
|
||||
|
Total operating expenses |
|
1,844,239 |
|
|
299,999 |
|
|
4,872,511 |
|
|
999,482 |
|
||||
|
|
|
|
|
|
|
|
|
|||||||||
|
Loss from operations |
|
(1,844,239 |
) |
|
(299,999 |
) |
|
(4,872,511 |
) |
|
(999,482 |
) |
||||
|
|
|
|
|
|
|
|
|
|||||||||
|
Other income (expense) |
|
|
|
|
|
|
|
|
||||||||
|
Change in fair value of warrant liability |
|
12,859 |
|
|
— |
|
|
22,377 |
|
|
— |
|
||||
|
Interest income |
|
56,426 |
|
|
116 |
|
|
71,676 |
|
|
2,794 |
|
||||
|
Interest expense |
|
(6,301 |
) |
|
(32,638 |
) |
|
(67,387 |
) |
|
(81,669 |
) |
||||
|
Total other income (expense) |
|
62,984 |
|
|
(32,522 |
) |
|
26,666 |
|
|
(78,875 |
) |
||||
|
|
|
|
|
|
|
|
|
|||||||||
|
Net loss |
$ |
(1,781,255 |
) |
$ |
(332,521 |
) |
$ |
(4,845,845 |
) |
$ |
(1,078,357 |
) |
||||
|
|
|
|
|
|
|
|
|
|||||||||
|
Net loss per common share – basic and diluted |
$ |
(0.14 |
) |
$ |
(0.04 |
) |
$ |
(0.47 |
) |
$ |
(0.14 |
) |
||||
|
Weighted average common shares outstanding |
|
12,575,983 |
|
|
7,903,850 |
|
|
10,308,911 |
|
|
7,903,850 |
|
||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-3
Apimeds Pharmaceuticals US, Inc.
Unaudited Condensed Statements of Changes in Stockholders’ Equity (Deficit)
|
Preferred Stock |
Common Stock |
Additional |
Accumulated |
Total |
|||||||||||||||||
|
Number of |
Amount |
Number of |
Amount |
||||||||||||||||||
|
Balance at December 31, 2024 |
— |
$ |
— |
7,903,850 |
$ |
79,039 |
$ |
2,954,764 |
$ |
(4,391,924 |
) |
$ |
(1,358,121 |
) |
|||||||
|
Net loss for the period ended March 31, 2025 |
— |
|
— |
— |
|
— |
|
— |
|
(402,397 |
) |
|
(402,397 |
) |
|||||||
|
Balance at March 31, 2025 |
— |
|
— |
7,903,850 |
|
79,039 |
|
2,954,764 |
|
(4,794,321 |
) |
|
(1,760,518 |
) |
|||||||
|
Stock-based compensation – stock options |
— |
|
— |
— |
|
— |
|
192,053 |
|
— |
|
|
192,053 |
|
|||||||
|
Stock-based compensation – common stock grants |
— |
|
— |
1,000,000 |
|
10,000 |
|
1,690,000 |
|
— |
|
|
1,700,000 |
|
|||||||
|
Conversion of convertible debt – related party |
— |
|
— |
297,133 |
|
2,971 |
|
496,251 |
|
— |
|
|
499,222 |
|
|||||||
|
Issuance of Representative Warrants in connection with IPO |
— |
|
— |
— |
|
— |
|
139,388 |
|
— |
|
|
139,388 |
|
|||||||
|
Issuance of common stock in IPO (net of $1,599,060 in offering costs and warrant liability) |
— |
|
— |
3,375,000 |
|
33,750 |
|
11,595,977 |
|
— |
|
|
11,629,727 |
|
|||||||
|
Net loss for the period ended June 30, 2025 |
— |
|
— |
— |
|
— |
|
— |
|
(2,662,193 |
) |
|
(2,662,193 |
) |
|||||||
|
Balance at June 30, 2025 |
— |
|
— |
12,575,983 |
|
125,760 |
|
17,068,433 |
|
(7,456,514 |
) |
|
9,737,679 |
|
|||||||
|
Stock-based compensation – stock options |
— |
|
— |
— |
|
— |
|
42,674 |
|
— |
|
|
42,674 |
|
|||||||
|
Issuance of Advisor Warrants in connection with IPO |
— |
|
— |
— |
|
— |
|
161,554 |
|
— |
|
|
161,554 |
|
|||||||
|
Net loss for the period ended September 30, 2025 |
— |
|
— |
— |
|
— |
|
— |
|
(1,781,255 |
) |
|
(1,781,255 |
) |
|||||||
|
Balance at September 30, 2025 |
— |
$ |
— |
12,575,983 |
$ |
125,760 |
$ |
17,272,661 |
$ |
(9,237,769 |
) |
$ |
8,160,652 |
|
|||||||
F-4
Apimeds Pharmaceuticals US, Inc.
Unaudited Condensed Statements of Changes in Stockholders’ Equity (Deficit) — (Continued)
|
Preferred Stock |
Common Stock |
Additional |
Accumulated |
Total |
|||||||||||||||||
|
Number of |
Amount |
Number of |
Amount |
||||||||||||||||||
|
Balance at December 31, 2023 |
— |
$ |
— |
7,903,850 |
$ |
79,039 |
$ |
2,954,764 |
$ |
(3,001,934 |
) |
$ |
31,869 |
|
|||||||
|
Net loss for the period ended March 31, 2024 |
— |
|
— |
— |
|
— |
|
— |
|
(296,473 |
) |
|
(296,473 |
) |
|||||||
|
Balance at March 31, 2024 |
— |
|
— |
7,903,850 |
|
79,039 |
|
2,954,764 |
|
(3,298,407 |
) |
|
(264,604 |
) |
|||||||
|
Net loss for the period ended June 30, 2024 |
— |
|
— |
— |
|
— |
|
— |
|
(449,363 |
) |
|
(449,363 |
) |
|||||||
|
Balance at June 30, 2024 |
— |
|
— |
7,903,850 |
|
79,039 |
|
2,954,764 |
|
(3,747,770 |
) |
|
(713,967 |
) |
|||||||
|
Net loss for the period ended September 30, 2024 |
— |
|
— |
— |
|
— |
|
— |
|
(332,521 |
) |
|
(332,521 |
) |
|||||||
|
Balance at September 30, 2024 |
— |
$ |
— |
7,903,850 |
$ |
79,039 |
$ |
2,954,764 |
$ |
(4,080,291 |
) |
$ |
(1,046,488 |
) |
|||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-5
Apimeds Pharmaceuticals US, Inc.
Unaudited Condensed Statements of Cash Flows
|
For the nine months ended |
||||||||
|
2025 |
2024 |
|||||||
|
Cash flows from operating activities: |
|
|
|
|
||||
|
Net loss |
$ |
(4,845,845 |
) |
$ |
(1,078,357 |
) |
||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
||||
|
Stock-based compensation – common stock grants |
|
1,700,000 |
|
|
— |
|
||
|
Stock-based compensation – stock options |
|
234,727 |
|
|
— |
|
||
|
Change in FV of warrant liability |
|
(22,377 |
) |
|
— |
|
||
|
Depreciation expense of property and equipment |
|
1,721 |
|
|
— |
|
||
|
Accrued interest expense – related parties |
|
27,554 |
|
|
26,568 |
|
||
|
Accretion expense |
|
39,833 |
|
|
55,101 |
|
||
|
|
|
|
|
|||||
|
Changes in operating assets and liabilities |
|
|
|
|
||||
|
Prepaid expenses and other current and non-current assets |
|
(2,219,630 |
) |
|
1,259 |
|
||
|
Accounts payable and accrued expenses |
|
(23,558 |
) |
|
361,519 |
|
||
|
Net cash used in operating activities |
|
(5,107,575 |
) |
|
(633,910 |
) |
||
|
|
|
|
|
|||||
|
Cash flows from investing activities: |
|
|
|
|
||||
|
Purchase of furniture and equipment |
|
(35,909 |
) |
|
— |
|
||
|
Net cash provided by investing activities |
|
(35,909 |
) |
|
— |
|
||
|
|
|
|
|
|||||
|
Cash flows from financing activities: |
|
|
|
|
||||
|
Cash proceeds from issuance of common stock in IPO |
|
11,953,046 |
|
|
— |
|
||
|
Proceeds from notes payable – related parties |
|
250,000 |
|
|
250,000 |
|
||
|
Cash advances from related parties |
|
17,400 |
|
|
— |
|
||
|
Cash advances paid to related parties |
|
(93,800 |
) |
|
— |
|
||
|
Net cash provided by financing activities |
|
12,126,646 |
|
|
250,000 |
|
||
|
|
|
|
|
|||||
|
Net increase (decrease) in cash |
|
6,983,162 |
|
|
(383,910 |
) |
||
|
Cash, beginning of period |
|
3,455 |
|
|
410,481 |
|
||
|
Cash, end of period |
$ |
6,986,617 |
|
$ |
26,571 |
|
||
|
|
|
|
|
|||||
|
Supplemental disclosure of cash flow information: |
|
|
|
|
||||
|
Cash paid for interest |
$ |
— |
|
$ |
— |
|
||
|
Cash paid for taxes |
$ |
— |
|
$ |
— |
|
||
|
|
|
|
|
|||||
|
Non-cash investing and financing activities: |
|
|
|
|
||||
|
Conversion of convertible debt – related party |
$ |
386,676 |
|
$ |
— |
|
||
|
Conversion of accrued interest expense for convertible debt – related party |
$ |
112,546 |
|
$ |
— |
|
||
|
Issuance of Representative Warrants in connection with IPO |
$ |
300,942 |
|
$ |
— |
|
||
The accompanying notes are an integral part of these unaudited condensed financial statements.
F-6
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
1. DESCRIPTION OF BUSINESS
Business Description
Apimeds Pharmaceuticals US, Inc. (the “Company” or “Apimeds”) was formed as a corporation in May 2020 and was incorporated in the State of Delaware. Apimeds is a clinical stage company that is in the process of seeking U.S. Food and Drug Administration (“FDA”) approval for Apitox, a proprietary intradermally administered bee venom-based toxin.
Apimeds Inc., the majority shareholder of the Company which is a subsidiary of Inscobee Inc. (“Apimeds Korea”), and the Company entered into license agreements, under which the Company was granted the right to continue any clinical trial, acquire the permits and approval necessary from the FDA and commercially develop and market Apitox within the United States (see notes 3). Apimeds completed a positive Phase 3 trial for the treatment of pain associated with osteoarthritis in 2018 and is now proceeding with the next steps for FDA approval. In the future, the Company plans to investigate potential uses for Apitox to treat pain associated with multiple sclerosis (“MS”), and intends to conduct non-registered corporate sponsored studies to identify appropriate MS patient populations. Apitox is currently marketed and sold by Apimeds Korea in South Korea (Republic of Korea) as “Apitoxin” for the treatment of osteoarthritis.
The success of the Company is dependent on obtaining the necessary regulatory approvals of its product candidates, marketing its products and achieving profitable operations. The continuation of the research and development activities and the commercialization of its products, if approved, are dependent on the Company’s ability to successfully complete these activities and to obtain additional financing through a combination of financing activities and operations. It is not possible to predict either the outcome of future research and development or commercialization programs, or the Company’s ability to fund these programs.
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The Company has prepared these unaudited condensed financial statements in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) promulgated by the Financial Accounting Standards Board (“FASB”). Except as disclosed herein, there have been no material changes in the information disclosed in the Notes to the Financial Statements included in the Annual Report for the year ended December 31, 2024 (the “Annual Report”). Accordingly, the unaudited condensed financial statements and related disclosures herein should be read in conjunction with the Annual Report.
As permitted under the SEC requirements for interim reporting, certain footnotes or other financial information have been condensed or omitted. These financial statements include all normal and recurring adjustments that are considered necessary for the fair presentation of results for the interim periods presented. Revenues, expenses, assets and liabilities can vary during each quarter of the year. Therefore, the results and trends in these interim financial statements may not be representative of those for the full year.
Liquidity
As of September 30, 2025, the Company had an accumulated deficit of $9,237,769. The Company incurred net losses of $1,781,255 and $4,845,845 for the three and nine months ended September 30, 2025, respectively, and expects to continue to incur substantial losses in the future. On May 12, 2025, the Company consummated its initial public offering (the “IPO”) of 3,375,000 shares of its common stock at a price of $4.00 per share, generating net cash proceeds to the Company of $11.9 million. Based on cash that is available for Company operations, together with the proceeds from the IPO, and projections of future Company operations, the Company believes that its cash will be sufficient to fund the Company’s current operating plan through at least the next twelve months from the date of issuance of the accompanying unaudited condensed financial statements.
F-7
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make certain estimates, judgements and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Significant estimates and assumptions made in the accompanying unaudited condensed financial statements include, but are not limited to, the determination of prepaid clinical development costs, stock-based compensation and estimates that are related to convertible instruments. Actual results could differ from those estimates, and such differences could be material to the financial statements.
Fair Value Measurement
The fair value of the Company’s financial assets and liabilities reflects management’s estimate of amounts that the Company would have received in connection with the sale of the assets or paid in connection with the transfer of the liabilities in an orderly transaction between market participants at the measurement date. In connection with measuring the fair value of its assets and liabilities, the Company seeks to maximize the use of observable inputs (market data obtained from independent sources) and to minimize the use of unobservable inputs (internal assumptions about how market participants would price assets and liabilities). The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
|
Level 1 — |
Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. |
|||
|
Level 2 — |
Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active. |
|||
|
Level 3 — |
Unobservable inputs based on the Company’s assessment of the assumptions that market participants would use in pricing the asset or liability. |
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
A financial asset or liability classification within the hierarchy is determined based on the lowest level input that is significant to the fair value measurement. The tables below summarize the fair values of our financial assets and liabilities as of September 30, 2025, and December 31, 2024:
|
Fair Value at |
|
|||||||||||
|
Level 1 |
Level 2 |
Level 3 |
||||||||||
|
Warrant Liability |
$ |
— |
$ |
— |
$ |
— |
$ |
— |
||||
|
Fair Value at |
|
|||||||||||
|
Level 1 |
Level 2 |
Level 3 |
||||||||||
|
Warrant Liability |
$ |
— |
$ |
— |
$ |
— |
$ |
— |
||||
The Company accounts for warrants as either equity-classified or liability-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC Topic 480, Distinguishing Liabilities from Equity (“ASC 480”) and FASB ASC Topic 815, Derivatives and Hedging (“ASC 815”). The assessment considers whether the warrants are freestanding financial instruments pursuant to ASC 480, meet the definition of a liability
F-8
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
pursuant to ASC 480, and whether the warrants meet all of the requirements for equity classification under ASC 815, including whether the warrants are indexed to the Company’s own common stock and whether the warrant holders could potentially require “net cash settlement” in a circumstance outside of the Company’s control, among other conditions for equity classification. This assessment, which requires the use of professional judgment, is conducted at the time of warrant issuance and as of each subsequent quarterly period end date while the warrants are outstanding.
For issued or modified warrants that meet all of the criteria for equity classification, the warrants are required to be recorded as a component of additional paid-in capital at the time of issuance. For issued or modified warrants that do not meet all the criteria for equity classification, the warrants are required to be liability classified and recorded at their initial fair value on the date of issuance and remeasured at fair value and each balance sheet date thereafter. Changes in the estimated fair value of the liability classified warrants are recognized as a non-cash gain or loss on the statements of operations. The fair value of the Representative Warrants and initial liability and fair value upon issuance related to Advisor Warrant (as defined below) was estimated using a Black Scholes valuation approach (see Note 9).
On September 5, 2023, the Company entered into a consulting agreement with certain advisor, under which, upon completion of the IPO, the Company would issue to advisor warrants to purchase a number of shares of common stock equal to 6% of the aggregate number of shares sold in the IPO (the “Advisor Warrants”). The Advisor Warrants were issued on August 5, 2025. Because the obligation to issue the Advisor Warrants became unconditional at the IPO close (May 12, 2025), the Company recorded a warrant liability at the IPO date fair value and remeasures that liability at each reporting date. Because the Advisor Warrants were issued as compensation for the IPO-related advisory services, the initial fair value recognized at the IPO date was recorded as an offering cost that reduced the additional paid-in capital as of May 12, 2025.
For the Company’s warrant liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3), the following table provides a reconciliation of the beginning and ending balance for each category therein, and gains or losses recognized during the three and nine months ended September 30, 2025:
|
Beginning Balance – December 31, 2024 |
$ |
— |
|
|
|
Advisor warrant liability incurred in connection with the IPO |
|
183,931 |
|
|
|
Re-measurement adjustments: |
|
|
||
|
Change in fair value of warrant liability |
|
(22,377 |
) |
|
|
Balance – August 5, 2025 |
$ |
161,554 |
|
|
|
Reclassification of warrants to equity classification |
|
(161,554 |
) |
|
|
Ending balance – September 30, 2025 |
$ |
— |
|
|
August 5, |
|||
|
Warrant Liability |
|||
|
Fair Value |
$ |
161,554 |
|
|
Valuation technique |
|
Black-Scholes options pricing model |
|
|
Significant unobservable unit |
|
Volatility and risk-free rates |
|
The warrant liability as of May 12, 2025 (IPO date), was valued utilizing the Black-Scholes options pricing model with the following inputs: $1.81 of stock price, 4.09% risk-free rate, 78.29% volatility, 0% dividend rate, and the expected term of 5 years. The warrant liability as of August 5, 2025, was valued utilizing the Black-Scholes options pricing model with the following inputs: $1.78 of stock price, 3.74% risk-free rate, 77.11% volatility, 0% dividend rate, and the expected term of 5 years. Upon issuance of the advisor warrants on August 5, 2025, the Advisor Warrants were reclassified to additional paid-in capital and will remain equity classified.
F-9
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Upon the issuance of the warrants on August 5, 2025, the final terms were evaluated, and the warrants met all conditions for equity classification under ASC 815-40. As a result, the warrants were revalued as of August 5, 2025 with the change in value reflected in the statement of operations. That amount was then reclassified to additional paid-in capital. No gain or loss was recognized in the consolidated statements of operations in connection with the reclassification.
The warrants are no longer subject to recurring fair value measurement following equity classification. Prior to issuance, changes in the fair value of the warrant liability were recorded in other income (expense). For the three and nine months ended September 30, 2025, the Company recognized a gain of $12,859 and $22,377, respectively, in other income (expense) for the change in fair value.
Common Stock Reverse Stock Split
On February 7, 2025, the Company’s board of directors (the “Board”) approved and implemented a reverse stock split at a ratio of 1-for-2.6, which provided that every 2.6 shares of its issued and outstanding common stock was automatically combined into one issued and outstanding share of common stock, without any change in the par value per share. All share and per share amounts in the accompanying unaudited condensed financial statements and footnotes have been retrospectively adjusted for the reverse split.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist of cash accounts in financial institutions which, at times, may exceed the federal depository insurance corporation limit of $250,000. As of September 30, 2025, the Company has not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Segment Information
The Company operates as a single operating and reportable segment, which aligns with the way the Chief Executive Officer, designated as the Chief Operating Decision Maker (CODM), evaluates performance and allocates resources. The Company is a clinical-stage entity focused on the development of a proprietary intradermally administered bee venom-based therapeutic. As of September 30, 2025, the Company has not generated any revenue and does not have any material long-lived assets. The CODM assesses the Company’s performance primarily through the analysis of operating expenses, specifically within key categories such as research and development and general and administrative expenses. Given the Company is in a pre-revenue stage, these expense categories serve as the primary financial drivers.
Financial information provided to and utilized by the CODM is consistent with the Company’s U.S. GAAP financial statements, including the Statements of Operations, which reflect the loss. A single management team reports directly to the CODM and oversees the entire business comprehensively. Resource allocation, performance evaluation, incentive setting, and forecasting activities are conducted at the corporate level using the financial statements and a unified budget. Accordingly, the Company does not evaluate performance by geographic area or product line, as it has not yet commenced commercial operations and has limited activity due to current liquidity and funding constraints. All operations are based in the United States of America, and all assets and operating expenses — including those related to research and development and general and administrative functions — are attributed to the Company’s single reportable segment.
Cash
The Company considers all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents. As of September 30, 2025 and December 31, 2024, the Company had no cash equivalents.
F-10
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Convertible Instruments
The Company evaluates and accounts for conversion options embedded in convertible instruments in accordance with ASC 815 “Derivatives and Hedging Activities”.
Applicable U.S. GAAP requires companies to bifurcate conversion options from their host instruments and account for them as free-standing derivative financial instruments according to certain criteria. The criteria include circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under other U.S. GAAP with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument.
The Company accounts for convertible instruments (when we have determined that the embedded conversion options should not be bifurcated from their host instruments) as follows: The Company records when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are accreted over the term of the related debt to their stated date of redemption.
If a security or instrument becomes convertible only upon the occurrence of a future event outside the control of the Company, or, is convertible from inception, but contains conversion terms that change upon the occurrence of a future event, then any contingent beneficial conversion feature is measured and recognized when the triggering event occurs and contingency has been resolved.
Patent Costs
All patent-related costs incurred in connection with filing and prosecuting patent applications are expensed as incurred due to the uncertainty about the recovery of the expenditure. Amounts incurred are classified as general and administrative expenses in the accompanying statements of operations.
Leases
The Company accounts for a contract as a lease when it has the right to direct the use of the asset for a period of time while obtaining substantially all of the asset’s economic benefits. The Company determines the initial classification and measurement of its right-of-use assets (“ROU”) and lease liabilities at the lease commencement date and thereafter if modified. ROU assets and liabilities are to be represented on the balance sheet at the present value of future minimum lease payments to be made over the lease term. The Company has elected as an accounting policy not to apply the recognition requirements in ASC 842, Leases (“ASC 842”) to short-term leases. Short-term leases are leases that have a term of 12 months or less and do not include an option to purchase the underlying asset that the Company is reasonably certain to exercise. The Company recognizes the lease payments for short-term leases on a straight-line basis over the lease term. As of September 30, 2025 and December 31, 2024, the Company did not have leases that qualified as ROU assets.
Property and Equipment, net
Property and equipment, net is stated at cost less accumulated depreciation. These assets are depreciated over their estimated useful lives of three to seven years using the straight-line method.
The Company adheres to ASC 360 “Property, Plant, and Equipment” and periodically evaluates whether current facts or circumstances indicate that the carrying value of its depreciable assets to be held and used may not be recoverable. If such circumstances are determined to exist, an estimate of undiscounted future cash flows produced by the long-lived
F-11
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
assets, or the appropriate grouping of assets, is compared to the carrying value to determine whether impairment exists. If an asset is determined to be impaired, the loss is measured based on the difference between the asset’s fair value and its carrying value. For long-lived assets, the estimate of fair value is based on various valuation techniques, including a discounted value of estimated future cash flows. The Company reports an asset to be disposed of at the lower of its carrying value or its fair value less costs to sell.
Related Parties
The Company follows ASC 850, “Related Party Disclosures” for the identification of related parties and disclosure of related party transactions.
General and Administrative
General and administrative expenses consist primarily of management personnel costs, professional service fees, and other general overhead and facility costs, including rent and insurance, which relate to the Company’s general and administrative functions.
Research and Development
Research and development expenses consist primarily of consulting, regulatory and manufacturing related costs, third-party license fees and external costs of vendors engaged to conduct preclinical development activities. These costs are expensed as incurred and non-refundable prepayments for goods or services that will be used or rendered for future research and development activities are deferred and capitalized in prepaid expenses and other current assets.
The Company enters into arrangements with contract research organizations in connection with pre-clinical and clinical trials. Such arrangements often provide for payment prior to commencing the project or based upon predetermined milestones throughout the period during which services are expected to be performed. As part of the process of preparing the Company’s financial statements, management is required to estimate prepaid and accrued clinical trial expenses. The date on which services commence, the level of services performed on or before a given date, and the cost of such services are often determined based on subjective judgments informed by the facts and circumstances known to management from the terms of the contract and the Company’s ongoing monitoring of service performance. The Company makes these judgments based upon the facts and circumstances known to management based on the terms of the contract and the Company’s ongoing monitoring of service performance.
In line with the guidance suggested under ASC 450, Contingencies and ASC 730, Research and Development, all research and development costs will be expensed as incurred. Development and regulatory milestone payments are accounted for by estimating the probability of milestone achievement.
Stock Based Compensation
The Company accounts for share-based compensation in accordance with the fair value recognition provision of FASB ASC 718, Compensation — Stock Compensation (“ASC 718”), which prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options, and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense in the unaudited condensed financial statements based on the estimated grant date fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period). The Company accounts for forfeitures as they occur. The Company classifies share-based compensation expense in its statements of operations in the same manner in which the award recipient’s cash compensation costs are classified.
F-12
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
The fair value of each employee and non-employee stock option grant is estimated on the date of grant using the Black-Scholes option-pricing model. The Company is a public company but has limited company-specific historical and implied volatility information. Therefore, it estimates its expected stock volatility based on implied volatility. The expected term of the Company’s stock options for employees has been determined utilizing the “simplified” method for awards. The risk-free interest rate is determined by reference to the U.S. Treasury yield curve. Expected dividend yield is zero based on the fact that the Company has never paid cash dividends and does not expect to pay any cash dividends in the foreseeable future.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences attributable to differences between carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax reporting purposes and for operating loss and tax credit carryforwards. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes.
The Company’s deferred tax assets and liabilities are measured using enacted tax rates expected to apply in the years in which these temporary differences are expected to be recovered or settled. A valuation allowance is recorded to reduce deferred tax assets if it is determined that it is more likely than not that all or a portion of the deferred tax asset will not be realized. The Company considers many factors when assessing the likelihood of future realization of deferred tax assets, including recent earnings results, expectations of future taxable income, carryforward periods available and other relevant factors. The Company records changes in the required valuation allowance in the period that the determination is made.
The Company assesses its income tax position and records tax benefits for all years subject to examination based upon management’s evaluation of the facts, circumstances and information available as of the reporting date. For those tax positions where it is more likely than not that a tax benefit will be sustained, the Company records the largest amount of tax benefit with a greater than 50% likelihood of being realized upon ultimate settlement with a taxing authority having full knowledge of all relevant information. For those income tax positions where it is not more likely than not that a tax benefit will be sustained, the Company does not recognize a tax benefit in the financial statements. The Company records interest and penalties related to uncertain tax positions, if applicable, as a component of income tax expense.
Basic and Diluted Loss per share
Basic loss per share data for each period presented is computed using the weighted average number of shares of common stock outstanding during each such period. Diluted net loss per share is computed by giving effect to all potential shares of common stock to the extent they are dilutive.
The following table sets forth the number of potential shares of common stock that have been excluded from basic net loss per share because their effect was anti-dilutive:
|
For the nine months ended |
||||
|
2025 |
2024 |
|||
|
Employee stock options |
589,871 |
213,692 |
||
|
Representative Warrants |
168,750 |
— |
||
|
Advisor Warrants |
202,500 |
— |
||
|
Convertible notes and interest |
— |
283,397 |
||
|
961,121 |
497,089 |
|||
F-13
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Emerging Growth Company
The Company is an emerging growth company, as defined in Section 2(a) of the Securities Act of 1993, as amended (the “Securities Act”), as modified by the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”), and it may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, reduced disclosure obligations regarding executive compensation in its periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved.
Further, Section 102(b)(1) of the JOBS Act allows emerging growth companies to delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act, until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that it (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these unaudited condensed financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
Recently Issued Accounting Pronouncements
The Company considers the applicability and impact of all Accounting Standard Updates (ASUs). ASUs not discussed in these unaudited condensed financial statements were assessed and determined to be either not applicable or are expected to have minimal impact on the financial statements.
In November 2024, the FASB issued Accounting Standards Update No. 2024-03, Disaggregation of Income Statement Expenses. This guidance will require additional disclosures and disaggregation of certain costs and expenses presented on the face of the income statement. The amendments are effective for annual reporting periods beginning after December 15, 2026 and interim reporting period beginning after December 15, 2027 with early adoption permitted. The Company is currently evaluating the impact of this new guidance to our financial statements.
3. LICENSE AGREEMENTS
On August 2, 2021, the Company entered into a business agreement with Apimeds Korea. Under the agreement, the Company received the right to continue any clinical trial and acquire the permits and approval necessary from the U.S. Food and Drug Administration. The Company will pay Apimeds Korea a royalty of 5% of the earnings before interest and taxes, delivered from the sale or license of Apitox less any credits and charges, however, the royalty terms shall not apply when shares of the Company are transferred or sold through merger, acquisition, or share transfer agreement to a third party.
On October 12, 2021, the Company entered into an exclusive patent license agreement with Apimeds Korea, a shareholder of the Company. Under the agreement, the Company was granted the exclusive right and license under the licensed patents to make and sell the licensed products in the United States of America.
The agreement commenced on the effective date and shall remain in force for each licensed product on a licensed-product-by-licensed-product basis for rights and obligations concerning the licensed patent, until the expiration of the last to expire valid claim of a licensed patent. The total consideration exchanged for the exclusive license agreement was $1.
F-14
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
4. PREPAID EXPENSE AND OTHER ASSETS
As of September 30, 2025, and December 31, 2024, the prepaid expense and other assets balance consists of the following:
|
September 30, |
December 31, |
||||||
|
Prepaid insurance |
$ |
344,990 |
|
$ |
— |
||
|
Prepaid clinical development costs |
|
1,729,886 |
|
|
— |
||
|
Other prepaid assets |
|
154,355 |
|
|
9,602 |
||
|
Less: long-term portion of prepaid insurance |
|
(129,740 |
) |
|
— |
||
|
Prepaid expenses and other current assets, current |
$ |
2,099,491 |
|
$ |
9,602 |
||
5. ACCOUNTS PAYABLE AND ACCRUED EXPENSE
Accounts payable and accrued expenses consist of balances owed to vendors, as well as others, such as the taxing authority and employees.
As of September 30, 2025, and December 31, 2024, the accounts payable and accrued expense balances consists of the following:
|
September 30, |
December 31, |
|||||
|
Professional fees payable |
$ |
193,377 |
$ |
410,641 |
||
|
Clinical trials payable |
|
352,349 |
|
— |
||
|
Accrued compensation |
|
18,250 |
|
180,550 |
||
|
Other |
|
3,657 |
|
— |
||
|
Total accounts payable and accrued expenses |
$ |
567,633 |
$ |
591,191 |
||
6. DEBT
2022 Convertible notes (amended from notes payable) — related parties
On March 21, 2022, the Company issued a promissory note in the amount of $160,000 to Inscobee, one of its shareholders. On June 3, 2022, the Company issued another $100,000 promissory note to Inscobee (together, and as amended, the “2022 Convertible Notes”). The 2022 Convertible Notes bear interest at 5% per annum and mature on the earlier of (a) the closing of an equity financing with proceeds to the Company of at least $3 million, or (b) July 15, 2022.
On December 5, 2023, the Company amended their promissory notes to be convertible and extended the maturity date of the convertible notes with the related parties to be the earlier of (i) December 31, 2026 or (ii) consummation of a qualified offering. The notes are convertible at a price of $1 per share. The purchase of convertible notes and cancellation of the old promissory notes was accounted for as a debt extinguishment that did not result in a gain/loss on extinguishment due to related party treatment. The conversion option was valued utilizing the Black-Scholes model, with the following inputs: volatility of 92.22%, current stock price of $1.96, expected dividend yield of 0% and a risk-free rate of return of 4.33%. The resulting value of the convertible option of $158,099 based on the allocation of relative fair value to cash proceeds, was applied towards additional paid-in capital and added as a discount on the convertible note. The note will be accreted over the remaining period through maturity at the calculated effective interest rate of approximately 41.4%.
In connection with the closing of the IPO, the 2022 Convertible Notes and 2021 Convertible Note (defined below) automatically converted into shares of Common Stock. Pursuant to the terms of the 2021 Convertible Note and 2022 Convertible Notes (as amended), all outstanding accrued and unpaid interest owed under the 2021 Convertible Note and 2022 Convertible Notes was to convert into common stock simultaneously with the consummation of an offering of common stock resulting in the listing of the Common Stock on the NYSE American, or other national securities exchange (a “Qualified Offering”). An aggregate of $660,000 outstanding principal together with $112,576 and
F-15
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
6. DEBT (cont.)
accrued interest under the 2021 Convertible Note and 2022 Convertible Notes was converted to Common Stock, resulting in the issuance of an aggregate of 297,133 shares of Company’s Common Stock, based on a conversion price of $2.60 per share, as set forth in the 2021 Convertible Note and 2022 Convertible Notes. As of the date of the conversion, the outstanding balances for the 2021 Convertible Note and 2022 Convertible Notes were $235,439 and $151,237, respectively, net of the unamortized debt discounts of $164,561 and $108,763. The total of unamortized debt discounts for the 2021 Convertible Note and 2022 Convertible Notes in the aggregate amount of $273,324 as of the date of the conversion was reflected within additional paid in capital, and the carrying aggregate amount of the 2021 Convertible Note and 2022 Convertible Notes of $386,676 along with accrued outstanding interest for the 2021 Convertible Note and 2022 Convertible Notes in the aggregate amount $112,576 as of the date of the conversion are reflected within condensed statement of changes in shareholders’ equity (deficit).
As of December 31, 2024, there was accrued interest in connection to the 2022 Convertible Notes of $34,745. Interest expenses were $1,498 and $4,596 for the three and nine months ended September 30, 2025, respectively. Interest expenses were $3,170 and $9,438 for the three and nine months ended September 30, 2024, respectively.
There was accretion on the note’s debt discount in connection to the 2022 Convertible Notes of $5,171 and $15,771 for the three and nine months ended September 30, 2025, respectively. There was accretion on the note’s debt discount of $8,898 and $21,742 for the three and nine months ended September 30, 2024, respectively.
2021 Convertible note — related party
On August 30, 2021, the Company issued a convertible promissory note in the amount of $400,000 (“2021 Convertible Note”) to Apimeds Korea. The 2021 Convertible Note bears interest at 5% per annum and matures on the earlier of (a) the sale of the Company or (b) August 30, 2026. The 2021 Convertible Note is convertible at any time up through the maturity date.
On December 5, 2023, the Company amended their convertible note to be convertible at $1 per share and extended the maturity date to be the earlier of (i) December 31, 2026 or (ii) consummation of a Qualified Offering. The repurchase and cancellation of the old note was accounted for as a debt extinguishment that did not result in any gain/loss on extinguishment due to related party treatment. The conversion option was valued utilizing the Black-Scholes model, with the following inputs: volatility of 92.22%, the fair value of the stock of $1.96, expected dividend yield of 0%, and a risk-free rate of return of 4.33%. The resulting value of the convertible option of $240,079, based on the allocation of relative fair value to cash proceeds, was applied towards additional paid-in capital and added as a discount on the convertible note. The note will be accreted over the remaining period through maturity at the calculated effective interest rate of approximately 40.6%.
In connection with the closing of the IPO, the 2022 Convertible Notes and 2021 Convertible Note automatically converted into shares of common stock (see 2022 Convertible notes (amended from notes payable) — related parties per above).
As of December 31, 2024, there was accrued interest in connection with the 2021 Convertible Note of $66,137 and is included within accrued interest — related party on the accompanying unaudited condensed balance sheets.
Interest expenses were $2,301 and $7,068 for the three and nine months ended September 30, 2025, respectively. Interest expenses were $4,877 and $14,521 for the three and nine months ended September 30, 2024, respectively.
There was accretion on the note’s debt discount in connection to the 2021 Convertible Notes of $7,884 and $24,061 for the three and nine months ended September 30, 2025, respectively. There was accretion on the note’s debt discount of $13,632 and $33,359 for the three and nine months ended September 30, 2024, respectively.
F-16
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
6. DEBT (cont.)
2024 Promissory Notes — Related Parties
On May 20, 2024, the Company issued a $100,000 promissory note to Inscobee. On August 19, 2024, the Company issued a $150,000 promissory note to Inscobee (together, the “2024 Promissory Notes”). The 2024 Promissory Notes bear interest at 5% per annum and mature on the earlier of (a) the closing of an equity financing by the Company with gross proceeds of at least $3,000,000; or (b) May 19, 2025. On May 16, 2025, the 2024 Promissory Notes were amended to extend the maturity date of for the outstanding principal and accrued interest payment date to May 19, 2026.
As of September 30, 2025 and December 31, 2024, there was accrued interest in connection with the 2024 Promissory Notes of $11,959 and $5,760. Interest expenses were $3,116 and $6,199 for the three and nine months ended September 30, 2025, respectively, and are included within accrued interest — related party on the accompanying unaudited condensed balance sheet. Interest expenses were $548 for the three and nine months ended September 30, 2024.
2025 Promissory Note — Related Parties
On March 21, 2025, the Company issued a $250,000 promissory note to Apimeds Korea (the “2025 Promissory Note”). The 2025 Promissory Note bears interest at 5% per annum and matures on the earlier of (a) December 31, 2026 or (b) consummation of a Qualified Offering. On May 16, 2025, the 2025 Promissory Note was amended to extend the maturity date of for the outstanding principal and accrued interest payment date to May 19, 2026.
As of September 30, 2025, there was accrued interest in connection with the 2025 Promissory Note of $3,390. Interest expenses were $3,082 and $3,390 for the three and nine months ended September 30, 2025, respectively, and are included within accrued interest — related party on the accompanying unaudited condensed balance sheet.
7. ADVANCE PAYABLE — RELATED PARTY
As of September 30, 2025, and December 31, 2024 the Company had an outstanding balance of $100 and $76,500, respectively, due to funds received from officers of the Company.
These advance payables carry no interest and do not have a maturity date. The cash proceeds from these advance payables were used for operating purposes.
8. COMMITMENTS AND CONTINGENCIES
Legal
Periodically, the Company reviews the status of any significant matters that exist and assesses its potential financial exposure. If the potential loss from any claim or legal claim is considered probable and the amount can be estimated, the Company accrues a liability for the estimated loss. Legal proceedings are subject to uncertainties, and the outcomes are difficult to predict. Because of such uncertainties, accruals are based on the best information available at the time. As additional information becomes available, the Company reassesses the potential liability related to pending claims and litigation. As of September 30, 2025 and December 31, 2024, there are no pending claims or litigation that are expected to materially affect the Company’s results going forward.
Executive employee agreement
On September 21, 2023, the Company signed an executive employee agreement with the Chief Executive Officer (CEO) of the Company. Under the executive employee agreement terms, if the Company closes on a public offering, the CEO will be eligible to receive an incentive stock option to purchase a number of shares of the Company’s common stock equal to 3% of the post-IPO capitalization of the Company. 40% of the options shall vest immediately upon grant and the remainder will vest in three equal installments on the annual anniversary of the date of grant.
F-17
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
8. COMMITMENTS AND CONTINGENCIES (cont.)
On May 12, 2025, the Company consummated the IPO. Immediately following the IPO on May 16, 2025, the Board approved the grant of 347,279 stock options to the CEO, with vesting terms of 40% on the grant date and the remaining 60% vesting in three equal annual installments on each anniversary of the grant date. In addition to the stock option grant, the Board also granted 750,000 shares of the Company’s Common Stock to the CEO of the Company, which are fully vested and unrestricted.
9. SHAREHOLDERS’ EQUITY
Common Stock
As of September 30, 2025 and December 31, 2024, the Company had 100,000,000 authorized shares of common stock. The Company had 12,575,983 and 7,903,850 shares of common stock issued and outstanding, as of September 30, 2025 and December 31, 2024, respectively. Each share of common stock is entitled to one vote.
On February 7, 2025, the Board approved and implemented a reverse stock split ratio of 1-for-2.6, which provided that every 2.6 shares of its issued and outstanding common stock were automatically combined into one issued and outstanding share of common stock, without any change in the par value per share. All share and per share amounts in the accompanying unaudited condensed financial statements and footnotes have been retrospectively adjusted for the reverse stock split.
On May 12, 2025, the Company consummated the IPO of 3,375,000 shares of its common stock at a price of $4.00 per share, generating net proceeds to the Company of $11.6 million after deducting underwriting discounts, offering expenses and the value of the Advisory Warrant liability. Out of the total shares issued, 500,000 shares were purchased by Inscobee.
In connection with the closing of the IPO, the 2022 Convertible Notes and 2021 Convertible Note automatically converted into shares of common stock. Pursuant to the terms of the 2021 Convertible Note and 2022 Convertible Notes, all outstanding accrued and unpaid interest owed under the 2021 Convertible Note and 2022 Convertible Notes was to convert into common stock simultaneously with the consummation of a Qualified Offering. An aggregate of $499,222 of outstanding principal and accrued interest under the 2022 Convertible Notes and 2021 Convertible Note, net of unamortized debt discount of $273,324, was converted to common stock, resulting in the issuance of an aggregate of 297,133 shares of Company’s common stock, based on a conversion price of $2.60 per share, as set forth in the 2021 Convertible Note and 2022 Convertible Notes.
Immediately following the IPO on May 16, the board of directors approved the grant of 750,000 and 250,000 shares of the Company’s common stock to the CEO and Chief Medical Officer of the Company, respectively. Such stock were issued under the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan (the “2024 Equity Incentive Plan”) and are fully vested and unrestricted. The value of the fully vested shares granted was determined by the value of the stock on the quoted trading price of $1.70 per share and in aggregate of $1,700,000, and recorded as stock-based compensation — stock grants, with $1,275,000 and $425,000 allocated to general and administrative expenses and research and development expenses, respectively, for the three and nine month periods ended September 30, 2025.
Warrants
In connection with the IPO, the Company entered into an Underwriting Agreement, dated May 8, 2025, between the Company and its underwriter. The Company also agreed to issue warrants to purchase an aggregate of 168,750 shares of common stock (the “Representative Warrants”), each dated May 12, 2025, to underwriter and its designees. The Representative Warrants have an exercise price of $5.00 per share and also feature a cashless exercise option. The initial exercise date of the Representative Warrants is November 4, 2025.
F-18
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
9. SHAREHOLDERS’ EQUITY (cont.)
The Company accounts for Representative Warrants as equity-classified instruments based on an assessment of the warrant’s specific terms and applicable authoritative guidance in FASB ASC Topic 480, Distinguishing Liabilities from Equity (“ASC 480”) and FASB ASC Topic 815, Derivatives and Hedging (“ASC 815”). The measurement of fair value of the Representative Warrants was determined utilizing a Black-Scholes model considering all relevant assumptions current at the date of issuance (i.e., share price of $1.81, exercise price of $5.00, term of 5 years, volatility of 78%, risk-free rate of 4.09%, and expected dividend rate of 0.0%). The grant date fair value of these Representative Warrants was estimated to be $139,388 on May 12, 2025, and was reflected as a reduction to additional paid-in capital as of May 12, 2025.
On September 5, 2023, the Company entered into a consulting agreement with certain advisor, under which, upon completion of the IPO, the Company would issue to advisor warrants to purchase a number of shares of common stock equal to 6% of the aggregate number of shares sold in the IPO (the “Advisor Warrants”). The Advisor Warrants were issued on August 5, 2025. Because the obligation to issue the Advisor Warrants became unconditional at the IPO close (May 12, 2025), the Company recorded a warrant liability at the IPO date fair value and remeasures that liability at each reporting date. Because the Advisor Warrants were issued as compensation for the IPO-related advisory services, the initial fair value recognized at the IPO date was recorded as an offering cost that reduced the additional paid-in capital as of May 12, 2025.
The warrant liability as of May 12, 2025 (IPO date), was valued utilizing the Black-Scholes options pricing model with the following inputs: $1.81 of stock price, 4.09% risk-free rate, 78.29% volatility, 0% dividend rate, and the expected term of 5 years. The warrant liability as of August 5, 2025, was valued utilizing the Black-Scholes options pricing model with the following inputs: $1.78 of stock price, 3.74% risk-free rate, 77.11% volatility, 0% dividend rate, and the expected term of 5 years.
Upon the issuance of the warrants on August 5, 2025, the final terms were evaluated, and the warrants met all conditions for equity classification under ASC 815-40. As a result, the warrants were revalued as of August 5, 2025 with the change in value reflected in the statement of operations. That amount was then reclassified to additional paid-in capital. No gain or loss was recognized in the consolidated statements of operations in connection with the reclassification.
Preferred Stock
On December 5, 2023, the Company authorized 10,000,000 shares of preferred stock with a par value of $0.01. The rights and preferences of preferred shareholders have not been determined as of the date of filing. The Company had no preferred shares issued or outstanding as of September 30, 2025, and December 31, 2024.
10. STOCK-BASED COMPENSATION
Stock Options
On September 18, 2024, the Company adopted the 2024 Equity Incentive Plan. 1,538,462 shares of common stock have initially been reserved for the issuance of awards under the 2024 Equity Incentive Plan with 42,283 shares available for future issuance as of September 30, 2025. There were 213,692 nonqualified stock option awards issued and outstanding outside of the 2024 Equity Incentive Plan as of September 30, 2025 and December 31, 2024.
The Company and its subsidiaries calculate stock-based compensation expense in accordance with ASC 718. The fair value of stock-based awards is amortized over the vesting period of the award.
There were 496,179 stock options granted under the 2024 Equity Incentive Plan to the Company’s employees and directors during the three and nine months ended September 30, 2025, and no stock options granted for three and nine months ended September 30, 2024.
F-19
Apimeds Pharmaceuticals US, Inc.
Notes to the Unaudited Condensed Financial Statements
10. STOCK-BASED COMPENSATION (cont.)
The stock options granted during the three and nine months ended September 30, 2025, were valued utilizing the Black-Scholes options pricing model with the following inputs: $1.70 - $1.93 of stock price, 4.06% risk-free rate, 78.23% – 81.85% volatility, 0% dividend rate, and the expected term of 5.50-6.00 years.
The following represents a summary of options:
|
Number of |
Weighted |
Weighted- |
|||||
|
Issued and outstanding, December 31, 2024 |
213,692 |
$ |
7.33 |
5.12 |
|||
|
Granted |
496,179 |
|
1.82 |
— |
|||
|
Exercised |
— |
|
— |
— |
|||
|
Forfeited/Expired |
— |
|
— |
— |
|||
|
Issued and outstanding, September 30, 2025 |
709,871 |
$ |
3.48 |
8.22 |
|||
|
Exercisable at September 30, 2025 |
372,604 |
$ |
5.22 |
6.76 |
|||
For the three and nine months ended September 30, 2025 the Company had $42,674 and $234,727 of stock compensation related to the stock options outstanding, of which $13,629 and $178,424 were included in general and administrative expenses and research and development expenses, respectively, on the accompanying unaudited condensed statements of operations. There was no expense related to the stock option grants recognized during the three and nine months ended September 30, 2024. As of September 30, 2025, the remaining unamortized expense of $372,805 will be recognized over the next 2.52 years. Such amount does not include the effect of future grants of equity compensation, if any. The intrinsic value of options outstanding was $1,445 at September 30, 2025 and the intrinsic value of options exercisable was $0 at December 31, 2024.
11. INCOME TAXES
The Company recorded no provision or benefit for income tax expense for the three and nine months ended September 30, 2025 and 2024, respectively.
For all periods presented, the pretax losses incurred by the Company received no corresponding tax benefit because the Company concluded that it is more likely than not that the Company will be unable to realize the value of any resulting deferred tax assets. The Company will continue to assess its position in future periods to determine if it is appropriate to reduce a portion of its valuation allowance in the future.
The Company has no open tax audits with any taxing authority as of September 30, 2025.
12. SUBSEQUENT EVENTS
The company’s management has evaluated subsequent events occurring after September 30, 2025, the date of our most recent balance sheet, through the date our financial statements were issued.
On October 15, 2025, the Company entered into a Waiver Agreement with D. Boral Capital in connection to its previously executed Underwriting Agreement dated May 8, 2025, whereby the Right of First Refusal (as defined in the Waiver Agreement) and the Company Lock-Up Agreements (as defined in the Waiver Agreement) are waived and terminated. In consideration for such waiver and termination, the Company paid to D. Boral Capital a non-refundable fee of $700,000 upon execution of the Waiver Agreement.
On October 15, 2025, the board of directors approved the grant of 510,500 options for shares of the Company’s common stock to participants in the 2024 Equity Incentive Plan and vest in quarterly installments beginning on the respective vesting commencement dates, such that the awards shall be fully vested after three years. The exercise price of the options granted was determined by the value of the stock on the quoted trading price of $1.92 per share.
F-20
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Apimeds Pharmaceuticals US, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Apimeds Pharmaceuticals US, Inc. as of December 31, 2024 and 2023, and the related statements of operations, changes in shareholders’ equity (deficit), and cash flows for each of the two years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Apimeds Pharmaceuticals US, Inc. as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the two years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the entity will continue as a going concern. As discussed in Note 1 to the financial statements, the entity has suffered recurring losses from operations and has accumulated deficit that raise substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the entity’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to Apimeds Pharmaceuticals US, Inc. in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Apimeds Pharmaceuticals US, Inc. is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Kreit & Chiu CPA LLP
We have served as Apimeds Pharmaceuticals US, Inc.’s auditor since 2023.
New York, New York
April 15, 2025
F-21
Apimeds Pharmaceuticals US, Inc.
Balance Sheets
|
December 31, |
December 31, |
|||||||
|
Assets |
|
|
|
|
||||
|
Current assets: |
|
|
|
|
||||
|
Cash |
$ |
3,455 |
|
$ |
410,481 |
|
||
|
Prepaid expenses and other current assets |
|
9,602 |
|
|
11,595 |
|
||
|
Total current assets |
|
13,057 |
|
|
422,076 |
|
||
|
Total assets |
$ |
13,057 |
|
$ |
422,076 |
|
||
|
|
|
|
|
|||||
|
Liabilities and shareholders’ equity (deficit) |
|
|
|
|
||||
|
Current liabilities: |
|
|
|
|
||||
|
Accounts payable and accrued expenses |
$ |
591,191 |
|
$ |
54,438 |
|
||
|
Accrued interest – related party |
|
106,643 |
|
|
68,878 |
|
||
|
Advance payable to related party |
|
76,500 |
|
|
— |
|
||
|
Notes payable – related party |
|
250,000 |
|
|
— |
|
||
|
Total current liabilities |
|
1,024,334 |
|
|
123,316 |
|
||
|
Convertible note – related party |
|
346,844 |
|
|
266,891 |
|
||
|
Total liabilities |
|
1,371,178 |
|
|
390,207 |
|
||
|
|
|
|
|
|||||
|
Commitments and contingencies (note 6) |
|
|
|
|
||||
|
|
|
|
|
|||||
|
Shareholders’ equity (deficit): |
|
|
|
|
||||
|
Preferred stock, par value $0.01, 10,000,000 shares authorized; none issued and outstanding as of December 31, 2024 and December 31, 2023 |
|
— |
|
|
— |
|
||
|
Common stock, par value $0.01, 100,000,000 shares authorized; 7,903,850 issued and outstanding as of December 31, 2024 and December 31, 2023 |
|
79,039 |
|
|
79,039 |
|
||
|
Additional paid-in capital |
|
2,954,764 |
|
|
2,954,764 |
|
||
|
Accumulated deficit |
|
(4,391,924 |
) |
|
(3,001,934 |
) |
||
|
Total shareholders’ equity (deficit) |
|
(1,358,121 |
) |
|
31,869 |
|
||
|
Total liabilities and shareholders’ equity (deficit) |
$ |
13,057 |
|
$ |
422,076 |
|
||
The accompanying notes are an integral part of these financial statements.
F-22
Apimeds Pharmaceuticals US, Inc.
Statements of Operations
|
For the year ended |
||||||||
|
2024 |
2023 |
|||||||
|
Operating expenses: |
|
|
|
|
||||
|
Research and development expenses |
$ |
— |
|
$ |
98,544 |
|
||
|
General and administrative expenses |
|
1,275,095 |
|
|
648,892 |
|
||
|
Loss from operations |
|
(1,275,095 |
) |
|
(747,436 |
) |
||
|
|
|
|
|
|||||
|
Other (expenses) income |
|
|
|
|
||||
|
|
|
|
|
|||||
|
Interest income |
|
2,824 |
|
|
7,811 |
|
||
|
Interest expense |
|
(117,719 |
) |
|
(38,069 |
) |
||
|
Total other expense |
|
(114,895 |
) |
|
(30,258 |
) |
||
|
Net loss |
$ |
(1,389,990 |
) |
$ |
(777,694 |
) |
||
|
Weighted average shares outstanding |
|
7,903,850 |
|
|
4,598,265 |
|
||
|
Basic and diluted loss per share |
$ |
(0.18 |
) |
$ |
(0.17 |
) |
||
The accompanying notes are an integral part of these financial statements.
F-23
Apimeds Pharmaceuticals US, Inc.
Statement of Changes in Shareholders’ Equity (Deficit)
|
Preferred Stock |
Common Stock |
Additional |
Accumulated |
Total |
|||||||||||||||||
|
Number of |
Amount |
Number of |
Amount |
||||||||||||||||||
|
Balance at December 31, 2022 |
— |
$ |
— |
3,846,154 |
$ |
38,462 |
$ |
1,472,172 |
$ |
(2,224,240 |
) |
$ |
(713,606 |
) |
|||||||
|
Stock-based compensation expense |
— |
|
— |
— |
|
— |
|
69,993 |
|
— |
|
|
69,993 |
|
|||||||
|
Issuance of shares to shareholders |
— |
|
— |
4,057,696 |
|
40,577 |
|
1,014,423 |
|
— |
|
|
1,055,000 |
|
|||||||
|
Embedded conversion feature of convertible notes |
— |
|
— |
— |
|
— |
|
398,176 |
|
— |
|
|
398,176 |
|
|||||||
|
Net loss |
— |
|
— |
— |
|
— |
|
— |
|
(777,694 |
) |
|
(777,694 |
) |
|||||||
|
Balance at December 31, 2023 |
— |
$ |
— |
7,903,850 |
$ |
79,039 |
$ |
2,954,764 |
$ |
(3,001,934 |
) |
$ |
31,869 |
|
|||||||
|
Net loss |
— |
|
— |
— |
|
— |
|
— |
|
(1,389,990 |
) |
|
(1,389,990 |
) |
|||||||
|
Balance at December 31, 2024 |
— |
$ |
— |
7,903,850 |
$ |
79,039 |
$ |
2,954,764 |
$ |
(4,391,924 |
) |
$ |
(1,358,121 |
) |
|||||||
The accompanying notes are an integral part of these financial statements.
F-24
Apimeds Pharmaceuticals US, Inc.
Statements of Cash flows
|
For the Years Ended |
||||||||
|
2024 |
2023 |
|||||||
|
Cash flows from operating activities: |
|
|
|
|
||||
|
Net loss |
$ |
(1,389,990 |
) |
$ |
(777,694 |
) |
||
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
||||
|
Stock-based compensation expense |
|
— |
|
|
69,993 |
|
||
|
Accrued interest expense – related parties |
|
37,766 |
|
|
33,000 |
|
||
|
Accretion expense |
|
79,953 |
|
|
5,069 |
|
||
|
Changes in operating assets and liabilities |
|
|
|
|
||||
|
Prepaid expenses and other current assets |
|
1,993 |
|
|
(11,594 |
) |
||
|
Accounts payable and accrued expenses |
|
536,752 |
|
|
53,436 |
|
||
|
Net cash used in operating activities |
|
(733,526 |
) |
|
(627,790 |
) |
||
|
|
|
|
|
|||||
|
Cash flows from investing activities: |
|
|
|
|
||||
|
Net cash provided by investing activities |
|
— |
|
|
— |
|
||
|
|
|
|
|
|||||
|
Cash flows from financing activities: |
|
|
|
|
||||
|
Proceeds from notes payable – related parties |
|
250,000 |
|
|
— |
|
||
|
Cash advances from related parties |
|
76,500 |
|
|
9,000 |
|
||
|
Cash advances paid to related parties |
|
— |
|
|
(31,900 |
) |
||
|
Issuance of shares for cash received |
|
— |
|
|
1,055,000 |
|
||
|
Net cash provided by financing activities |
|
326,500 |
|
|
1,032,100 |
|
||
|
|
|
|
|
|||||
|
Net (decrease) increase in cash |
|
(407,026 |
) |
|
404,310 |
|
||
|
Cash, beginning of year |
|
410,481 |
|
|
6,171 |
|
||
|
Cash, end of year |
$ |
3,455 |
|
$ |
410,481 |
|
||
The accompanying notes are an integral part of these financial statements.
F-25
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
1. DESCRIPTION OF BUSINESS
Business Description
Apimeds Pharmaceuticals US, Inc. (the “Company” or “Apimeds”) was formed as a corporation in May 2020 and was incorporated in the State of Delaware. On August 21, 2021, Apimeds Inc., the shareholder of the Company (“Apimeds Korea”), and Apimeds Pharmaceuticals US Inc. entered into the business agreement, under which the Company was designated to operate a pharmaceutical business which provides the biological drug named Apitox™ to clients in the biological drug commercial transaction area.
Apimeds is a clinical stage company that is in the process of developing Apitox™, a proprietary intradermally administered bee venom-based toxin which completed a positive Phase 3 trial for the treatment of pain associated with Osteoarthritis in 2018 and is now proceeding with FDA discussions on next steps in approval. In the future, the Company plans to investigate potential uses for Apitox™ for in treating multiple sclerosis (“MS”), and intends to conduct non-registered corporate sponsorship studies to identify appropriate MS patient populations. Apitox™ is currently marketed and sold by Apimeds Korea in South Korea (Republic of Korea) as “Apitoxin” for the treatment of osteoarthritis. Apimeds Inc. holds the majority of the Company’s outstanding common stock and is a subsidiary of Inscobee Inc. (“Inscobee”).
The success of the Company is dependent on obtaining the necessary regulatory approvals of its product candidates, marketing its products and achieving profitable operations. The continuation of the research and development activities and the commercialization of its products, if approved, are dependent on the Company’s ability to successfully complete these activities and to obtain additional financing through a combination of financing activities and operations. It is not possible to predict either the outcome of future research and development or commercialization programs, or the Company’s ability to fund these programs.
Going Concern
The Company has evaluated whether there are any conditions and events, considered in the aggregate, that raise substantial doubt about its ability to continue as a going concern within one year beyond the issuance date of these financial statements. As of December 31, 2024, the Company had accumulated losses amount to $4,391,924. The Company incurred net losses of $1,389,990 for the year ended December 31, 2024, and expects to continue to incur substantial losses in the future. Based on such conditions and the Company’s current plans, which are subject to change, management believes that the Company’s existing cash as of December 31, 2024, is not sufficient to satisfy its operating cash needs for 12 months from the issuance date of the report
The accompanying financial statements have been prepared assuming the Company will continue to operate as a going concern, which contemplates the realization of assets and settlement of liabilities in the normal course of business, and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classifications of liabilities that may result from uncertainty related to its ability to continue as a going concern.
If the Company is unable to obtain sufficient financial resources, its business, financial condition and results of operations will be materially and adversely affected. This could affect future development and business activities and potential future clinical studies and/or other future ventures. There can be no assurance that the Company will be able to obtain the needed financing on acceptable terms or at all.
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The Company has prepared its financial statements in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASUs”) promulgated by the Financial Accounting Standards Board (“FASB”).
F-26
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make certain estimates, judgements and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Significant estimates and assumptions made in the accompanying financial statements include, but are not limited to, stock-based compensation and estimates that are related to convertible instruments. Actual results could differ from those estimates, and such differences could be material to the financial statements.
Fair Value Measurement
The fair value of the Company’s financial assets and liabilities reflects management’s estimate of amounts that the Company would have received in connection with the sale of the assets or paid in connection with the transfer of the liabilities in an orderly transaction between market participants at the measurement date. In connection with measuring the fair value of its assets and liabilities, the Company seeks to maximize the use of observable inputs (market data obtained from independent sources) and to minimize the use of unobservable inputs (internal assumptions about how market participants would price assets and liabilities). The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
|
Level 1 — |
Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis. |
|||
|
Level 2 — |
Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active. |
|||
|
Level 3 — |
Unobservable inputs based on the Company’s assessment of the assumptions that market participants would use in pricing the asset or liability. |
In some circumstances, the inputs used to measure fair value might be categorized within different levels of the fair value hierarchy. In those instances, the fair value measurement is categorized in its entirety in the fair value hierarchy based on the lowest level input that is significant to the fair value measurement.
Common Stock Reverse Stock Split
On February 7, 2025, the Board approved and implemented a reverse stock split ratio of 1-for-2.6, which provided that every 2.6 shares of its issued and outstanding Common Stock was automatically be combined into one issued and outstanding share of Common Stock, without any change in the par value per share. All share and per share amounts in the accompanying financial statements and footnotes have been retrospectively adjusted for the reverse split.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentration of credit risk consist of cash accounts in financial institutions which, at times, may exceed the federal depository insurance corporation limit of $250,000. As of December 31, 2024, the Company has not experienced losses on these accounts and management believes the Company is not exposed to significant risks on such accounts.
Segment Information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker (“CODM”), or decision-making group, in making decisions on how to allocate resources and assess performance. The Company has one operating segment.
F-27
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Cash
The Company considers all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash equivalents. As of December 31, 2024 and 2023, the Company had no cash equivalents.
Accrued Expenses
Accrued expenses consist of accrued interest for the convertible and promissory notes held with related parties, monies owed to vendors, as well as others, such as the taxing authority and employees.
As December 31, 2024, and 2023, the accounts payable and accrued expenses balance consists of the following:
|
As of December 31, |
||||||
|
2024 |
2023 |
|||||
|
Professional fees payable |
$ |
410,641 |
$ |
54,438 |
||
|
Accrued compensation |
|
180,550 |
|
— |
||
|
$ |
591,191 |
$ |
54,438 |
|||
Convertible Instruments
The Company evaluates and accounts for conversion options embedded in convertible instruments in accordance with ASC 815 “Derivatives and Hedging Activities”.
Applicable U.S. GAAP requires companies to bifurcate conversion options from their host instruments and account for them as free-standing derivative financial instruments according to certain criteria. The criteria include circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under other U.S. GAAP with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument.
The Company accounts for convertible instruments (when we have determined that the embedded conversion options should not be bifurcated from their host instruments) as follows: The Company records when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are accreted over the term of the related debt to their stated date of redemption.
If a security or instrument becomes convertible only upon the occurrence of a future event outside the control of the Company, or, is convertible from inception, but contains conversion terms that change upon the occurrence of a future event, then any contingent beneficial conversion feature is measured and recognized when the triggering event occurs and contingency has been resolved.
Patent Costs
All patent-related costs incurred in connection with filing and prosecuting patent applications are expensed as incurred due to the uncertainty about the recovery of the expenditure. Amounts incurred are classified as general and administrative expenses in the accompanying statements of operations.
Leases
The Company accounts for a contract as a lease when it has the right to direct the use of the asset for a period of time while obtaining substantially all of the asset’s economic benefits. The Company determines the initial classification and measurement of its right-of-use assets (“ROU”) and lease liabilities at the lease commencement date and thereafter if
F-28
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
modified. ROU assets and liabilities are to be represented on the balance sheet at the present value of future minimum lease payments to be made over the lease term. The Company has elected as an accounting policy not to apply the recognition requirements in ASC 2016-02, Leases (“ASC 842”) to short-term leases. Short-term leases are leases that have a term of 12 months or less and do not include an option to purchase the underlying asset that the Company is reasonably certain to exercise. The Company recognizes the lease payments for short-term leases on a straight-line basis over the lease term. As of December 31, 2024 and 2023, the Company did not have leases that qualified as ROU assets.
Related Parties
The Company follows ASC 850, “Related Party Disclosures” for the identification of related parties and disclosure of related party transactions.
General and Administrative
General and administrative expenses consist primarily of management personnel costs, professional service fees, and other general overhead and facility costs, including rent and insurance, which relate to the Company’s general and administrative functions.
Research and Development
Research and development expenses consist primarily of consulting, regulatory and manufacturing related costs, third-party license fees and external costs of vendors engaged to conduct preclinical development activities. These costs are expensed as incurred and non-refundable prepayments for goods or services that will be used or rendered for future research and development activities are deferred and capitalized in prepaid expenses and other current assets.
The Company enters into arrangements with contract research organizations in connection with pre-clinical and clinical trials. Such arrangements often provide for payment prior to commencing the project or based upon predetermined milestones throughout the period during which services are expected to be performed. As part of the process of preparing the Company’s financial statements, management is required to estimate prepaid and accrued clinical trial expenses. The date on which services commence, the level of services performed on or before a given date, and the cost of such services are often determined based on subjective judgments informed by the facts and circumstances known to management from the terms of the contract and the Company’s ongoing monitoring of service performance. The Company makes these judgments based upon the facts and circumstances known to management based on the terms of the contract and the Company’s ongoing monitoring of service performance.
In line with the guidance suggested under ASC 450, Contingencies and ASC 730, Research and Development, all research and development costs will be expensed as incurred. Development and regulatory milestone payments are accounted for by estimating the probability of milestone achievement.
Stock Based Compensation
The Company accounts for share-based compensation in accordance with the fair value recognition provision of FASB ASC 718, Compensation — Stock Compensation (“ASC 718”), which prescribes accounting and reporting standards for all share-based payment transactions in which employee services are acquired. Transactions include incurring liabilities, or issuing or offering to issue shares, options, and other equity instruments such as employee stock ownership plans and stock appreciation rights. Share-based payments to employees, including grants of employee stock options, are recognized as compensation expense in the financial statements based on the estimated grant date fair values. That expense is recognized over the period during which an employee is required to provide services in exchange for the award, known as the requisite service period (usually the vesting period). The Company accounts for forfeitures as they occur. The Company classifies share-based compensation expense in its statements of operations in the same manner in which the award recipient’s cash compensation costs are classified.
F-29
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Given the absence of an active market for the Company’s equity, the Company and the board of directors were required to estimate the fair value of the Company’s common stock and equity awards at the time of each grant. The Company and the board of directors determined the estimated fair value of the Company’s equity instruments based on a number of factors, including external market conditions affecting the pharmaceutical industry sector. The Company and the board of directors utilized various valuation methodologies in accordance with the framework of the American Institute of Certified Public Accountants’ Technical Practice Aid, Valuation of Privately Held Company Equity Securities Issued as Compensation, to estimate the fair value of its equity instrument. Each valuation methodology includes estimates and assumptions that require the Company’s judgment.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences attributable to differences between carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax reporting purposes and for operating loss and tax credit carryforwards. Changes in deferred tax assets and liabilities are recorded in the provision for income taxes.
The Company’s deferred tax assets and liabilities are measured using enacted tax rates expected to apply in the years in which these temporary differences are expected to be recovered or settled. A valuation allowance is recorded to reduce deferred tax assets if it is determined that it is more likely than not that all or a portion of the deferred tax asset will not be realized. The Company considers many factors when assessing the likelihood of future realization of deferred tax assets, including recent earnings results, expectations of future taxable income, carryforward periods available and other relevant factors. The Company records changes in the required valuation allowance in the period that the determination is made.
The Company assesses its income tax position and records tax benefits for all years subject to examination based upon management’s evaluation of the facts, circumstances and information available as of the reporting date. For those tax positions where it is more likely than not that a tax benefit will be sustained, the Company records the largest amount of tax benefit with a greater than 50% likelihood of being realized upon ultimate settlement with a taxing authority having full knowledge of all relevant information. For those income tax positions where it is not more likely than not that a tax benefit will be sustained, the Company does not recognize a tax benefit in the financial statements. The Company records interest and penalties related to uncertain tax positions, if applicable, as a component of income tax expense.
Basic and Diluted Loss per share
Basic loss per share data for each period presented is computed using the weighted average number of shares of common stock outstanding during each such period. Diluted net loss per share is computed by giving effect to all potential shares of common stock to the extent they are dilutive.
The following table sets forth the number of potential shares of common stock that have been excluded from basic net loss per share because their effect was anti-dilutive:
|
As of December 31, |
||||
|
2024 |
2023 |
|||
|
Employee stock options |
211,538 |
211,538 |
||
|
Convertible notes and interest |
294,863 |
280,337 |
||
|
506,401 |
491,875 |
|||
F-30
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
2. BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Emerging Growth Company
The Company intends to elect as an Emerging Growth Company, as defined in Section 2(a) of the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act, until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that it (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
Prior period reclassifications
We have reclassified certain amounts in prior periods to conform with current presentation. Accrued interest — related party in the amount of $68,878, was reported within accounts payable and accrued expenses at December 31, 2023, amd have been reclassified on the balance sheet and statement of cash flows.
Recently Issued Accounting Pronouncements
The Company considers the applicability and impact of all Accounting Standard Updates. ASUs not discussed in these financial statements were assessed and determined to be either not applicable or are expected to have minimal impact on the financial statements.
In November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2023-07 — Segment Reporting (ASC 280): Improvements to Reportable Segment Disclosures, which enables investors to better understand an entity’s overall performance and assess potential future cash flows through improved reportable segment disclosure requirements. The amendments enhance disclosures about significant segment expenses, clarify circumstances in which an entity can disclose multiple segment measures of profit or loss, provide new segment disclosure requirements for entities with a single reportable segment, and contain other disclosure requirements. ASU 2023-07 is effective for annual periods beginning after December 15, 2023. The Company adopted ASU No. 2023-07 on December 31, 2024. The adoption of the standard did not result in any significant disclosure changes in the Notes to the Financial Statements.
In December 2023, the FASB issued ASU No. 2023-09, Income Taxes — Improvements to Income Tax Disclosures (Topic 740). The amendments require that public business entities on an annual basis disclose specific categories in the rate reconciliation and provide additional information for reconciling items that meet a quantitative threshold. The amendments also require that all entities disclose on an annual basis the income taxes paid disaggregated by jurisdiction. The amendments eliminate the requirement for all entities to disclose the nature and estimate of the range of the reasonably possible change in the unrecognized tax benefits balance in the next 12 months or make a statement that an estimate of the range cannot be made. The amendments are effective for fiscal years beginning after December 15, 2024. The amendments should be applied on a prospective basis, although early adoption is permitted. The Company is currently evaluating the potential impact adopting ASU 2023-09 will have on the Company’s financial statements and related disclosures.
In November 2024, the FASB issued Accounting Standards Update No. 2024-03, Disaggregation of Income Statement Expenses. This guidance will require additional disclosures and disaggregation of certain costs and expenses presented on the face of the income statement. The amendments are effective for annual reporting periods beginning after December 15, 2026 and interim reporting period beginning after December 15, 2027 with early adoption permitted. The Company is currently evaluating the impact of this new guidance to our financial statements.
F-31
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
3. LICENSE AGREEMENTS
On August 2, 2021, the Company entered into a business agreement with Apimeds Korea. Under the agreement, the Company received the right to continue any clinical trial and acquire the permits and approval necessary from the U.S. Food and Drug Administration. The Company will pay Apimeds Korea a royalty of 5% of the earnings before interest and taxes, delivered from the sale or license of Apitox less any credits and charges, however, the royalty terms shall not apply when shares of the Company are transferred or sold through merger, acquisition, or share transfer agreement to a third party.
On October 12, 2021, the Company entered into an exclusive patent license agreement with Apimeds Korea, a shareholder of the Company. Under the agreement, the Company was granted the exclusive right and license under the licensed patents to make and sell the licensed products in the United States of America.
The agreement shall commence on the effective date and shall remain in force for each licensed product on a licensed-product-by-licensed-product basis for rights and obligations concerning the licensed patent, until the expiration of the last to expire valid claim of a licensed patent. The total consideration exchanged for the exclusive license agreement was $1.
4. DEBT
2022 Convertible notes (amended from notes payable) — related parties
On March 21, 2022, the Company entered into a promissory note agreement in the amount of $160,000 with Inscobee, one of its shareholders. On June 3, 2022, the Company received an additional $100,000 from Inscobee, as part of another promissory note agreement (together as “2022 Convertible Notes”). The 2022 Convertible Notes bear interest at 5% per annum and mature on the earlier of (a) the closing of an equity financing with proceeds to the Company of at least $3 million, or (b) July 15, 2022.
On December 5, 2023, the Company amended their promissory notes to be convertible and extended the maturity date of the convertible notes with the related parties to be the earlier of (i) December 31, 2026 or (ii) consummation of a qualified offering. The notes are convertible at a price of $1 per share. The purchase of convertible notes and cancellation of the old promissory notes was accounted for as a debt extinguishment that did not result in a gain/loss on extinguishment due to related party treatment. The conversion option was valued utilizing the Black-Scholes model, with the following inputs: volatility of 92.22%, current stock price of $1.96, expected dividend yield of 0% and a risk-free rate of return of 4.33%. The resulting value of the convertible option of $158,099 based on the allocation of relative fair value to cash proceeds, was applied towards additional paid-in capital and added as a discount on the convertible note. The note will be accreted over the remaining period through maturity at the calculated effective interest rate of approximately 41.4%.
As of December 31, 2024 and 2023, there was accrued interest in connection to the 2022 Convertible Notes of $34,745 and $22,137, respectively. Interest expenses were $12,608 and $13,000 for the years ended December 31, 2024 and 2023, respectively, and are included within accrued interest — related party on the accompanying balance sheet. There was accretion on the note’s debt discount of $31,569 and $1,997 for the years ended December 31, 2024 and 2023.
As of December 31, 2024 and 2023, the outstanding balance on the 2022 Convertible notes agreement, net of the unamortized debt discounts of $124,534 and $156,102, was $135,466 and $103,898, respectively.
2021 Convertible note — related party
On August 30, 2021, the Company received $400,000 in a convertible note agreement (“2021 Convertible Note”) with Apimeds Korea, one of its shareholders. The 2021 Convertible Note bears interest at 5% per annum and matures on the earlier of (a) the sale of the Company or (b) August 30, 2026. The 2021 Convertible Note is convertible at any time up through the maturity date. The number of shares of common stock shall be determined by dividing (x) the outstanding principal balance hereof plus accrued but unpaid interest by the first closing price on the first day of trading following a Qualified Direct Listing.
F-32
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
4. DEBT (cont.)
On December 5, 2023, the Company amended their convertible note to be convertible at $1 per share and extended the maturity date to be the earlier of (i) December 31, 2026 or (ii) consummation of a qualified offering. The repurchase and cancellation of the old note was accounted for as a debt extinguishment that did not result in any gain/loss on extinguishment due to related party treatment. The conversion option was valued utilizing the Black-Scholes model, with the following inputs: volatility of 92.22%, the fair value of the stock of $1.96, expected dividend yield of 0%, and a risk-free rate of return of 4.33%. The resulting value of the convertible option of $240,079, based on the allocation of relative fair value to cash proceeds, was applied towards additional paid-in capital and added as a discount on the convertible note. The note will be accreted over the remaining period through maturity at the calculated effective interest rate of approximately 40.6%.
As December 31, 2024 and 2023, there was accrued interest in connection with the 2021 Convertible Note of $66,137 and $46,740, respectively, and is included within accrued interest — related party on the accompanying unaudited condensed balance sheets. Interest expense was $19,397 and 20,000 as of December 31, 2024 and 2023, respectively. Accretion on the 2021 Convertible Note discount was $48,385 for year ended December 31, 2024 respectively, which is included within interest expense on the unaudited condensed statement of operations. There was accretion on the 2021 Convertible Note debt discount of $48,385 and $3,072 for the years ended December 31, 2024 and 2023.
As of December 31, 2024 and 2023, the outstanding balance on the 2021 Convertible Note, net of the unamortized debt discounts of $188,622 and $237,007, was $211,378 and $162,993, respectively.
2024 Promissory Notes — Related Parties
On May 20, 2024, the Company received $100,000 in a promissory note agreement with Inscobee Inc., one of its shareholders. On Aug 19, 2024, the Company received an additional $150,000 from Inscobee, as part of another promissory note agreement (together as “2024 Promissory Notes”). The 2024 Promissory Notes bear interest at 5% per annum and mature on the earlier of (a) the closing of an equity financing by the Company with gross proceeds of at least $3,000,000; or (b) May 19, 2025.
As of December 31, 2024, there was accrued interest in connection with the 2024 Promissory Notes of $5,760. Interest expense was $5,760 for the year ended December 31, 2024, and is included within accrued interest — related party on the accompanying unaudited condensed balance sheet.
2024 Short Term Borrowing
On July 19, 2024, the Company entered into a non-interest-bearing loan agreement with a private lender for $20,000. The note matured on August 31, 2024, or may be extended upon mutual agreement. This loan was paid off in full on August 27, 2024.
5. ADVANCE PAYABLE — RELATED PARTY
As of December 31, 2024, the Company received $76,500 from an officer of the Company that is outstanding as of the year ended December 31, 2024.
In March 2023, the Company received $9,000 from the officer and remitted $31,900 back to the officer, leaving a net balance of $22,900 as of December 31, 2023.
These advance payables carry no interest and do not have a maturity date. The cash proceeds from these advance payables were used for operating purposes.
F-33
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
6. COMMITMENTS AND CONTINGENCIES
Legal
Periodically, the Company reviews the status of any significant matters that exist and assesses its potential financial exposure. If the potential loss from any claim or legal claim is considered probable and the amount can be estimated, the Company accrues a liability for the estimated loss. Legal proceedings are subject to uncertainties, and the outcomes are difficult to predict. Because of such uncertainties, accruals are based on the best information available at the time. As additional information becomes available, the Company reassesses the potential liability related to pending claims and litigation. As of December 31, 2024 and 2023, there are no pending claims or litigation that are expected to materially affect the Company’s results going forward.
Executive employee agreement
On September 21, 2023, the Company signed an executive employee agreement with the CEO of the Company. Under the executive employee agreement terms, if the Company closes on a public offering, the CEO will be eligible to receive an incentive stock option to purchase a number of shares of the Company’s common stock equal to 3% of the post-Public Offering capitalization of the Company. 40% of the options shall vest immediately upon grant and the remainder will vest in three equal installments on the annual anniversary of the date of grant.
7. SHAREHOLDERS’ DEFICIT
Common Stock
As of December 31, 2024 and 2023, the Company had 100,000,000 authorized shares of common stock, respectively, at a par value of $0.01. The Company had 7,903,850 common shares issued and outstanding, as of December 31, 2024 and 2023, respectively. Each Common share is entitled to one vote.
On February 7, 2025, the Board approved and implemented a reverse stock split ratio of 1-for-2.6, which provided that every 2.6 shares of its issued and outstanding Common Stock were automatically combined into one issued and outstanding share of Common Stock, without any change in the par value per share. All share and per share amounts in the accompanying financial statements and footnotes have been retrospectively adjusted for the reverse split
Preferred Stock
On December 5, 2023, the Company authorized 10,000,000 shares of preferred stock with a par value of $0.01. The rights and preferences of preferred shareholders have not been determined as of the date of filing. The Company had no preferred shares issued or outstanding as of the year ended December 31, 2024 and 2023, respectively.
Activity during the period ended December 31, 2023
On September 7, 2023, the Company issued 1,923,076 shares of common stock of the Company to related parties for cash consideration in aggregate of $500,000.
On December 5, 2023, the Company established a preferred stock class by authorizing 10,000,000 shares with a par value of $0.01.
On December 6, 2023, the Company issued 2,134,616 shares of common stock of the Company to related parties for cash consideration in aggregate of $555,000.
F-34
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
8. STOCK-BASED COMPENSATION
Stock Options
On September 18, 2024, the Company adopted an equity incentive plan for its employees, the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan (the “2024 Equity Incentive Plan”). 1,000,000 shares of common stock have initially been reserved for the issuance of awards under the 2024 Equity Incentive Plan with no stock options granted or outstanding as of the issuance date of the financial statements.
On May 12, 2020, the Company granted one of its executive officers a total of 213,692 nonqualified stock option awards issued outside of the 2024 Equity Incentive Plan. The stock options vested in three equal tranches of 71,231 on the grant anniversary date through May 12, 2023. The shares have an exercise price of $7.33 per share and expire in 10 years on May 12, 2030.
The following is a summary of stock options issued and outstanding as of December 31, 2024 and 2023:
|
Number of |
Weighted |
Weighted |
Aggregate |
||||||
|
Outstanding as of December 31, 2023 |
213,692 |
$ |
7.33 |
6.37 |
— |
||||
|
Granted |
— |
|
— |
— |
— |
||||
|
Exercised |
— |
|
— |
— |
— |
||||
|
Forfeited |
— |
|
— |
— |
— |
||||
|
Outstanding as of December 31, 2024 |
213,692 |
$ |
7.33 |
5.36 |
— |
||||
|
Exercisable as of December 31, 2024 |
213,692 |
$ |
7.33 |
5.36 |
— |
||||
During the years ended December 31, 2024 and 2023, there was $0 and $69,993, respectively, of stock-based compensation recognized.
The options were valued utilizing the Black-Scholes options pricing model with the following inputs: 0.20% risk-free rate, 66.8% volatility, 0% dividend rate, vesting term of 3 years, and the expected term of 6.5 years. The total fair value of shares vested during each of the years ended December 31, 2023 was $69,993.
As of December 31, 2024, there were no remaining unrecognized compensation costs related to unvested options.
9. INCOME TAXES
There were no income tax expenses reflected in the results of operations for the years ended December 31, 2024 and 2023.
|
Year Ended December 31, |
||||||||
|
2024 |
2023 |
|||||||
|
Net loss per book |
$ |
(1,389,990 |
) |
$ |
(777,694 |
) |
||
|
Federal statutory income tax rate (21%) |
|
(291,899 |
) |
|
(163,315 |
) |
||
|
State income tax, net of federal benefit |
|
(62,455 |
) |
|
(32,268 |
) |
||
|
State rate change |
|
— |
|
|
34,245 |
|
||
|
Permanent item |
|
16,888 |
|
|
1,083 |
|
||
|
Prior period adjustment |
|
(3,228 |
) |
|
3,549 |
|
||
|
Change in valuation allowance |
|
340,694 |
|
|
156,706 |
|
||
|
Income tax |
$ |
— |
|
$ |
— |
|
||
F-35
Apimeds Pharmaceuticals US, Inc.
Notes to Financial Statements
9. INCOME TAXES (cont.)
The tax effects of temporary differences which give rise to deferred tax assets (liabilities) are summarized as follows:
|
Year Ended December 31, |
||||||||
|
2024 |
2023 |
|||||||
|
Net operating loss carry forwards |
$ |
741,321 |
|
$ |
537,878 |
|
||
|
Stock-based compensation |
|
151,750 |
|
|
172,436 |
|
||
|
Accrued compensation |
|
182,509 |
|
|
— |
|
||
|
Capitalized research and development |
|
66,117 |
|
|
90,501 |
|
||
|
Intangible assets |
|
(824 |
) |
|
(638 |
) |
||
|
Total deferred tax assets |
|
1,140,873 |
|
|
800,177 |
|
||
|
Valuation allowance |
|
(1,140,873 |
) |
|
(800,177 |
) |
||
|
Net deferred tax assets |
$ |
— |
|
$ |
— |
|
||
The Company had cumulative federal net operating losses of approximately $2.85 million and state net operating losses of approximately $2.76 million, which do not expire but are subject to an 80% utilization against future taxable income.
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Deferred tax assets consist primarily of the tax effect of NOL carry-forwards. The Company has provided a full valuation allowance on the deferred tax assets because of the uncertainty regarding its realizability.
The Company’s policy is to record interest and penalties associated with unrecognized tax benefits as additional income taxes in the statement of operations. As of December 31, 2024, the Company had no unrecognized tax benefits. There were no changes in the Company’s unrecognized tax benefits during the years ended December 31, 2024 and 2023. The Company did not recognize any interest or penalties during the 2024 fiscal year related to unrecognized tax benefits.
10. SUBSEQUENT EVENTS
The Company evaluated subsequent events through the issuance date of the financial statements and determined that there have been no subsequent events except those mentioned throughout the footnotes that would require recognition in the financial statements or disclosure in the notes to the financial statements.
Subsequent to the year ended December 31, 2024, the Company received an additional $17,000 from an officer of the Company as advance payable to the related patty.
March 2025 Promissory Note
On March 31, 2025, the Company received $250,000 in a promissory note agreement with Apimeds, Inc., one of its shareholders. The Promissory Notes bear interest at 5% per annum and mature on the earlier of (a) December 31, 2026 or (b) consummation of a Qualified Offering (the “Maturity Date”). “Qualified Offering” shall mean an offering of Common Stock (and other securities potentially) resulting in the listing for trading of the Common Stock on the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market, the Nasdaq Global Select Market or the New York Stock Exchange (or any successors to any of the foregoing).
The Company may prepay the March 2025 Note at any time without penalty. If any payment due on the March 2025 Note is not paid within five days after the amount becomes due, the payment shall be considered in default and the interest rate will increase by an additional 5% on the defaulted payment amount and may also, in its sole discretion, without notice or demand, declare the entire unpaid principal balance plus accrued interest due and payable immediately.
F-36
MINDWAVE INNOVATIONS INC.
Consolidated Balance Sheets
(Unaudited)
|
September 30, |
March 31, |
|||||
|
ASSETS |
|
|
||||
|
Current assets: |
|
|
||||
|
Cash and cash equivalents |
$ |
6 |
$ |
6 |
||
|
Digital assets, at fair value |
|
3,300 |
|
— |
||
|
Total current assets |
|
3,306 |
|
6 |
||
|
Digital assets, at fair value |
|
132,273,773 |
|
138,865,011 |
||
|
Property and equipment, net |
|
— |
|
826 |
||
|
Total assets |
$ |
132,277,079 |
$ |
138,865,843 |
||
|
|
|
|||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
||||
|
Liabilities: |
$ |
— |
$ |
— |
||
|
|
|
|||||
|
Stockholders’ equity: |
|
|
||||
|
Common stock, 1,500 shares authorized, $0.01 par, 1,001 shares issued and outstanding as of both September 30, 2025 and March 31, 2025 |
|
10 |
|
10 |
||
|
Additional paid-in capital |
|
82,969,083 |
|
82,969,083 |
||
|
Retained earnings |
|
49,307,986 |
|
55,896,750 |
||
|
Total stockholders’ equity |
|
132,277,079 |
|
138,865,843 |
||
|
Total liabilities and stockholders’ equity |
$ |
132,277,079 |
$ |
138,865,843 |
||
See accompanying notes to consolidated financial statements.
F-37
MINDWAVE INNOVATIONS INC.
Consolidated Statements of Operations
(Unaudited)
|
Six Months Ended |
||||||||
|
2025 |
2024 |
|||||||
|
Revenue |
$ |
— |
|
$ |
— |
|
||
|
|
|
|
|
|||||
|
Operating expense (income): |
|
|
|
|
||||
|
General and administrative |
|
3,371,133 |
|
|
2,273,557 |
|
||
|
Distribution and commission expenses |
|
4,634,172 |
|
|
3,261,257 |
|
||
|
Trading gains, net |
|
(3,300 |
) |
|
— |
|
||
|
Realized gain on sale of digital assets |
|
(10,479,790 |
) |
|
(7,333,122 |
) |
||
|
Unrealized (gain) loss from changes in fair value of digital assets |
|
9,066,549 |
|
|
(56,026,550 |
) |
||
|
Total operating expense (income) |
|
6,588,764 |
|
|
(57,824,858 |
) |
||
|
|
|
|
|
|||||
|
(Loss) income from operations |
|
(6,588,764 |
) |
|
57,824,858 |
|
||
|
|
|
|
|
|||||
|
Provision for income taxes |
|
— |
|
|
— |
|
||
|
Net (loss) income |
$ |
(6,588,764 |
) |
$ |
57,824,858 |
|
||
|
|
|
|
|
|||||
|
Net (loss) income per share – basic and diluted |
$ |
(6,582.18 |
) |
$ |
57,767.09 |
|
||
|
Weighted average common shares outstanding – basic and diluted |
|
1,001 |
|
|
1,001 |
|
||
See accompanying notes to consolidated financial statements.
F-38
MINDWAVE INNOVATIONS INC.
Consolidated Statements of Stockholders’ Equity
(Unaudited)
|
|
Additional |
Retained |
Total |
|||||||||||||
|
Number |
Amount |
|||||||||||||||
|
Balances at March 31, 2024 |
1,001 |
$ |
10 |
$ |
2,713 |
$ |
(1,607 |
) |
$ |
1,116 |
|
|||||
|
Issuance of equity shares (see Note 7) |
— |
|
— |
|
528,510 |
|
— |
|
|
528,510 |
|
|||||
|
Net income |
— |
|
— |
|
— |
|
57,824,858 |
|
|
57,824,858 |
|
|||||
|
Balances at September 30, 2024 |
1,001 |
$ |
10 |
$ |
531,223 |
$ |
57,823,251 |
|
$ |
58,354,484 |
|
|||||
|
|
|
|
|
|
|
|||||||||||
|
Balances at March 31, 2025 |
1,001 |
$ |
10 |
$ |
82,969,083 |
$ |
55,896,750 |
|
$ |
138,865,843 |
|
|||||
|
Net loss |
— |
|
— |
|
— |
|
(6,588,764 |
) |
|
(6,588,764 |
) |
|||||
|
Balances at September 30, 2025 |
1,001 |
$ |
10 |
$ |
82,969,083 |
$ |
49,307,986 |
|
$ |
132,277,079 |
|
|||||
See accompanying notes to consolidated financial statements.
F-39
MINDWAVE INNOVATIONS INC.
Consolidated Statements of Cash Flows
(Unaudited)
|
Six Months Ended |
||||||||
|
2025 |
2024 |
|||||||
|
Cash flows from operating activities: |
|
|
|
|
||||
|
Net (loss) income |
$ |
(6,588,764 |
) |
$ |
57,824,858 |
|
||
|
Adjustments to reconcile net income to net cash (used in) provided by operating activities: |
|
|
|
|
||||
|
Unrealized (gain) loss from changes in fair value of digital assets |
|
9,066,549 |
|
|
(56,026,550 |
) |
||
|
Realized gain from sale of digital assets |
|
(10,479,790 |
) |
|
(7,333,122 |
) |
||
|
Depreciation |
|
— |
|
|
142 |
|
||
|
Property and equipment written off |
|
826 |
|
|
— |
|
||
|
Changes in operating assets and liabilities: |
|
|
|
|
||||
|
Digital assets |
|
(3,300 |
) |
|
— |
|
||
|
Net cash used in operating activities |
|
(8,004,479 |
) |
|
(5,534,672 |
) |
||
|
Cash flows from investing activities: |
|
|
|
|
||||
|
Sale consideration received as digital assets (net of expenses) |
|
(2,527,730 |
) |
|
(1,860,073 |
) |
||
|
Sale of digital assets |
|
10,532,210 |
|
|
7,394,745 |
|
||
|
Net cash provided by investing activities |
|
8,004,479 |
|
|
5,534,671 |
|
||
|
Net change in cash |
|
— |
|
|
(1 |
) |
||
|
Cash at beginning of period |
|
6 |
|
|
7 |
|
||
|
Cash at end of period |
$ |
6 |
|
$ |
6 |
|
||
|
|
|
|
|
|||||
|
Supplemental disclosure of cash flow information: |
|
|
|
|
||||
|
Cash paid for interest |
$ |
— |
|
$ |
— |
|
||
|
Cash paid for taxes |
$ |
— |
|
$ |
— |
|
||
|
|
|
|
|
|||||
|
Supplemental disclosure to investing and financing activities: |
|
|
|
|
||||
|
Non-cash purchase of digital assets in exchange of equity shares issued |
$ |
— |
|
$ |
528,510 |
|
||
See accompanying notes to consolidated financial statements.
F-40
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1: NATURE OF OPERATIONS
Mindwave Innovations Inc. (the “Company”) was incorporated under the laws of the State of Delaware on November 10, 2025 to become the ultimate holding company of TechyTrade FZ-LLC, a company incorporated in United Arab Emirates, and TechyTrade Innovations Pte. Ltd, a company incorporated in Singapore. TechyTrade Innovations Pte. Ltd. (“TechyTrade Singapore”) was incorporated in Singapore on September 8, 2025. TechyTrade FZ-LLC was originally a company incorporated in United Arab Emirates on December 27, 2020 and is the entity that maintains historical operations of the Company.
The Company is a leading provider of institutional Digital Asset Treasury (DAT) solutions, specializing in compliant Bitcoin treasury infrastructure, AI-driven yield capabilities, ClimateTech impact systems, and AdTech engagement platforms. The company’s multi-vertical ecosystem is powered by its native token, $NILA, which enables governance, utility, and value flow across its blockchain-integrated operations.
MindWave is a global leader in AI-driven Bitcoin and yield-generation technologies, operating in one of the fastest-growing segments of the digital-asset market. Bitcoin remains the most established and highly valued cryptocurrency, and MindWave’s platform is designed to help institutions securely hold, manage, and generate yield from Bitcoin reserves.
MindWave’s three-pronged strategic framework includes:
1. Secure Digital Treasury Wallets for Corporations,
2. AI-Enhanced Bitcoin Yield Generation, and
3. A Validator-Powered Ecosystem supported by the $NILA Token. Together, these capabilities make
MindWave one of the first companies to pursue a publicly traded, institutional-focused Digital Asset Treasury (DAT) model.
Reorganization
Prior to the reorganization transaction described below, TechyTrade FZ-LLC maintained historical operations of the Company since its formation in 2020. In September 2025, TechyTrade FZ-LLC became a wholly owned subsidiary of TechyTrade Innovations Pte. Ltd.
On November 10, 2025, Mindwave Innovations Inc. entered into a Reorganization Agreement and Plan of Share Exchange to consolidate its corporate structure. Under this agreement, the Company issued 1,001 shares of its common stock in exchange for 100% of the membership interests in TechyTrade FZ-LLC, which was previously held by TechyTrade Singapore. As a result of this transaction, TechyTrade FZ-LLC became a wholly owned subsidiary of Mindwave Innovations Inc., establishing the Company as the ultimate holding entity for the group.
The Reorganization is being accounted for as a reorganization of entities under common control. The accompanying financial statements have been presented to retroactively present the effect of the Reorganization. See Note 3 for further detail.
NOTE 2: LIQUIDITY AND CASH RESOURCES
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
The Company’s primary sources of liquidity have been cash flows from operating in sale of digital tokens. For the six months ended September 30, 2025 and 2024, the Company reported loss from operations of $6,588,764 and income from operations of $57,824,858, respectively. The company has no liability to be settled to the outsiders.
F-41
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 2: LIQUIDITY AND CASH RESOURCES (cont.)
Management has evaluated the Company’s ability to continue as a going concern within one year after the date the financial statements are issued, as required under ASC 205-40. Based on this evaluation, management has concluded that there are no conditions or events that raise substantial doubt about the Company’s ability to continue as a going concern. Accordingly, no material uncertainties have been identified that would cast significant doubt on the Company’s ability to meet its obligations as they become due.
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America (“US GAAP”).
The Company’s fiscal year is March 31.
The financial statements included herein comprise the consolidated results of the Company and its subsidiaries. All intercompany transactions have been eliminated. The consolidated financial statements represent the historical operations of TechyTrade FZ-LLC, which was incorporated in United Arab Emirates and the initial capital structure of Mindwave Innovations Inc., which was completed upon the Reorganization (see Note 1).
In accordance with ASC 805-50-15-6, the Company determined that the share exchange was a reorganization of entities under common control. Mindwave Innovations Inc. and TechyTrade FZ-LLC maintained common control for the entire period for which the financial statements are presented through the Reorganization. The Company concluded that the entities were under common control via common ownership and common management. Therefore, in accordance with ASC 250-10-45 and ASC 805-50-45, the financial statements require retrospective consolidation of the entities for all periods presented. The financial statements for the six months ended September 30, 2025 and 2024 are prepared on a consolidated basis of the Company and TechyTrade FZ-LLC.
The consolidated financial statements as of and for the six months ended September 30, 2025 and 2024 have been presented to reflect the historical operations of the Company for the periods presented. The financial statements reflect the revenues, expenses, assets, and liabilities to reflect all historical results of operations of the business.
Use of Estimates
The preparation of the accompanying financial statements in conformity with GAAP requires management to make certain estimates and assumptions that affect the reported amounts and disclosures of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. These estimates are based on information available through the date of the issuance of the financial statements and actual results could differ from those estimates. Estimates are adjusted to reflect actual experience when necessary. Significant estimates made by management include, but are not limited to:
• revenue recognition
• credit loss on accounts receivables and contract receivables
• provisions for income taxes and related valuation allowances and tax uncertainties
• recoverability of long-lived assets and their related estimated lives
• impairment of crypto currencies
• accruals for estimated liabilities
F-42
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Cash and Cash Equivalents
Cash and cash equivalents include all cash in banks. The Company considers all highly liquid instruments with original maturities of three months or less to be cash equivalents.
Concentration of Credit Risk
The Fund maintains its cash with a bank located in the United Arab Emirates and believes it to be creditworthy. Balances are covered by the local deposit insurance regulations as established by the Central Bank of the UAE. At times, the Fund may maintain balances in excess of these regulatory coverage limits.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and impairments. Normal repairs and maintenance costs are charged to earnings as incurred and additions and major improvements are capitalized. The cost of assets retired or otherwise disposed of, and the related depreciation are eliminated from the accounts in the period of disposal and the resulting gain or loss is credited or charged to earnings.
Depreciation is computed over the estimated useful lives of the related asset type using the straight-line method for financial statement purposes. The estimated useful lives for property and equipment consist of computer and equipment and straight-line depreciation is based on a useful life of 5 years.
Fair Value of Financial Instruments
We determine fair value measurements for certain assets and liabilities in accordance with Accounting Standards Codification Topic 820, Fair Value Measurements (“ASC 820”), which defines fair value as the exit price that would be received to sell an asset or paid to transfer a liability in an ordinary transaction between market participants. ASC 820 establishes a framework for valuation techniques, prioritized by reliability, according to the following tiers:
|
Level 1 — |
Unadjusted quoted prices in active markets for identical assets and liabilities |
|||
|
Level 2 — |
Quoted prices for similar assets and liabilities in active markets; quoted prices for similar or identical assets and liabilities in markets that are not active; valuation models in which all significant inputs are derived from observable market data |
|||
|
Level 3 — |
Unobservable valuation model inputs for assets and liabilities such as discounted cash flow models or similar techniques; inputs for fair value instruments; includes assumptions and may require significant judgment and estimation by management |
We use this framework to measure the fair value of certain financial instruments on a recurring basis, such as our cash and cash equivalents and digital assets at fair value, as well as on a non-recurring basis for our acquisitions and impairment testing on our property and equipment. See Note 4 — Fair Value Measurements for further discussion.
Net Income per Share
Net earnings per share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period, excluding shares subject to redemption or forfeiture. The Company presents basic and diluted net earnings or loss per share. Diluted net earnings or loss per share reflect the actual weighted average of common shares issued and outstanding during the period, adjusted for potentially dilutive securities outstanding. Potentially dilutive securities are excluded from the computation of the diluted net income per share if their inclusion would be anti-dilutive. As of September 30, 2025 and March 31, 2025, there were no potentially dilutive shares outstanding.
F-43
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Digital Assets
Digital assets are considered intangible assets under Accounting Standards Codification Subtopic 350-60, Intangibles — Goodwill and Other — Crypto Assets (“ASC 350-60”) and Accounting Standards Update 2023-08. Under ASC 350-60, digital assets that meet the definition of intangible assets, among other criteria, are initially recorded at cost and are then subsequently remeasured at fair value, as of the balance sheet date with changes from remeasurement recognized in net income. The fair value for digital assets that are unrestricted (“liquid”) and freely tradable are determined using Level 1 inputs as defined in ASC 820. Conversely, fair value for digital assets that are restricted (“locked”) due to vesting schedules or contractual restrictions are determined based on Level 1 inputs adjusted for the impact of such restrictions based on Level 2 inputs, in accordance with ASC 820. We account for changes from remeasurement within (gain) loss from changes in fair value of digital assets, as stated on our statements of operations.
Digital assets that we hold that do not meet the criteria under ASC 350-60 are accounted for as intangible assets with indefinite useful lives and are initially recorded at cost and are not amortized but instead are tested for impairment at least annually, or more frequently, if events or changes in circumstances indicate that it is more likely than not that the asset is impaired. As such, these digital assets are reflected in our balance sheets, at cost net of any impairments within Digital assets, at carrying value. We evaluate impairments on these digital assets on at least an annual basis, or more frequently if events or changes in circumstances occur and use Level 1 inputs, unadjusted quoted prices in active markets, for the fair value to determine if an impairment has occurred. If unadjusted quoted prices in active markets for our digital assets are lower than the carrying value recorded, then we will record an impairment charge equal to the difference between the carrying value and the fair value. Impairment charges are recognized within (Gain) loss on changes in fair value of digital assets, as stated on our statements of operations.
When we receive digital assets earned on third party staking platforms, we will initially record them at fair value on the date the digital assets are received. Upon the disposal of digital assets, any gains or losses are recognized in realized gain on sale of digital assets, as stated on our statements of operations, and are calculated as the difference between the selling price and our carrying value, using the specific identification method. See Note 6 — Digital Assets for further discussion.
Market-making Activities
The Company accounts for its market-making activities in accordance with ASC 350-60, Crypto Assets. Digital assets are measured at fair value at each reporting date, with changes in fair value recognized in operating income. The Company classifies these assets as Level 1 within the fair value hierarchy per ASC 820, utilizing unadjusted quoted prices from the Lbank exchange.
In accordance with ASC 350-60-45-1, realized gains and losses from trading are presented as trading (gains) losses, net within operating income.
Impairment of Long-lived Assets
Long-lived assets, such as property, equipment, and identifiable intangibles with finite useful lives, are periodically evaluated for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company looks for indicators of a trigger event for asset impairment and pays special attention to any adverse change in the extent or manner in which the asset is being used or in its physical condition. Assets are reviewed using factors including, but not limited to, future operating plans and projected cash flows. The determination of whether impairment has occurred is based on an estimate of undiscounted future cash flows directly related to the assets, compared to the carrying value of the assets. If the sum of the undiscounted future cash flows of the assets does not exceed the carrying value of the assets, full or partial impairment may exist. If the asset carrying amount exceeds its fair value, an impairment charge is recognized in the amount by which the carrying amount exceeds the fair value of the asset. Fair value is determined using an income approach, which requires discounting the estimated future cash flows associated with the asset.
F-44
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
As of September 30, 2025 and March 31, 2025, there was no impairment for long-lived assets.
Revenue Recognition
We recognize revenue in accordance with the five-step model outlined in Accounting Standard Codification Topic 606 — Revenue from Contracts with Customers (“ASC 606”). This model helps us identify the contract we have with our customers, identify the performance obligation in the contract, determine the transaction price, allocate the transaction price to the performance obligation and recognize revenue as the performance obligation is satisfied. We earn revenue from consulting services to customers.
During the six months ended September 30, 2025, the Company has not generated any revenue form from consulting services. The Company had realized and unrealized gains (losses) related to digital assets (see Note 6).
During the year ended September 30, 2024, the Company has not generated any revenue form from consulting services. The Company had realized and unrealized gains (losses) related to digital assets (see Note 6).
All revenue activity, and digital asset activity, were originated in the United Arab Emirates.
Cost of Revenue
Cost of revenue includes direct costs and costs related to marketing and technical consultancy.
Contract Balances
The Company does not typically record contract assets or contract liabilities, as consideration for token sales is received at or near the time control is transferred. No material contract asset or deferred revenue balances existed as of the reporting date unless otherwise disclosed.
Market Risk
The Company is exposed to market risk related to the digital asset holdings, which are impacted by the market value of the respective underlying digital asset. The Company performed a sensitivity analysis assuming a hypothetical 10% change in the fair value of these digital assets to demonstrate the potential impact on the financial results. A hypothetical 10% increase or decrease in market prices would have positively or negatively impacted Income (loss) before income taxes by approximately $13.2 million for the six months ended September 30, 2025.
General and Administrative
General and administrative expenses primarily consist of administrative costs and marketing and promotional expenses related to digital assets. Pursuant to a service agreement (see Note 10), the Company receives legal, administrative support, compliance (KYC/AML), reporting and security services for the underlying NILA tokens. These costs are expensed as incurred.
Distribution and Commission
Distribution and commission expenses primarily consist of deduction and payment of 44% of gross token sale proceeds as commission to the Distributor. Pursuant to a service agreement (see Note 10), the Distributor is committed to exclusive sales and collection of NILA tokens. These costs are expensed as incurred.
F-45
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Concentrations
In 2025, nearly all of the Company’s general and administrative and sales and marketing expenses were incurred to pursuant to one service agreement (see Note 10). The Company is dependent on two third-party vendors for its NILA token digital asset services and operations. The termination of this service agreement may have a negative short-term impact on the Company’s operations; however, the Company believes there are acceptable substitute vendors that can be utilized longer-term.
Segment Reporting
ASC Topic 280, “Segment Reporting,” establishes standards for companies to report in their financial statement information about operating segments, products, services, geographic areas, and major customers. Operating segments are defined as components of an enterprise for which separate financial information is available that is regularly evaluated by the Company’s chief operating decision maker, or group, in deciding how to allocate resources and assess performance.
Foreign Currency Translation
For the six months ended September 30, 2025 and 2024, the functional and reporting currency is the U.S. Dollar (“USD”).
Related Parties
Related parties are any entities or individuals that, through employment, ownership or other means, possess the ability to direct or cause the direction of the management and policies of the Company. The Company discloses related party transactions that are outside of normal compensatory agreements, such as loans. The Company follows ASC 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions.
Income Taxes
The Fund is organized as a Limited Partnership (LP) and is treated as a pass-through entity for U.S. federal income tax purposes. Accordingly, the Fund itself is generally not subject to U.S. federal income tax on its income; instead, the Fund’s taxable income or loss is allocated to the Partners who are responsible for their own tax obligations. The Fund may be subject to income or excise taxes in certain state and local jurisdictions. The Fund was historically a juridical entity in the UAE. Following the introduction of the UAE Corporate Tax (CT) regime, the Fund has determined that it meets the requirements to be treated as a Qualifying Investment Fund (QIF) or similar exempt status, and is therefore not subject to the UAE CT on its core investment income. Management has evaluated the Fund’s tax positions and determined there are no material uncertain tax positions requiring recognition as of the reporting date.
Recently Issued and Adopted Accounting Pronouncements
In July 2025, Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2025-05 Financial Instruments — Credit Losses (“ASU 2025-05”), which provides a practical expedient that can be elected to be applied to accounts receivable and contract assets, which would allow entities to assume that current conditions as of the balance sheet date do not change for the remaining life of the assets when estimating expected credit losses for such assets. ASU 2025-05 applies to public entities with annual reporting periods beginning after December 15, 2025, and interim reporting periods within those annual reporting periods. Early adoption is permitted in both interim and annual reporting periods in which financial statements have not yet been issued or made available for issuance. We are evaluating the impact this guidance may have on our financial statements.
F-46
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
In November 2024, FASB issued ASU No. 2024-03, Income Statement — Reporting Comprehensive Income — Expense Disaggregation Disclosures (“ASU 2024-03”), which enhances transparency by requiring public entities to disclose more detailed information about their income statement expenses. This includes disaggregating specific natural expense categories, like employee compensation and depreciation, within certain expense captions. ASU 2024-03 applies to public entities with annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027, early adoption is permitted. We are evaluating the impact this amended guidance may have on the notes to our condensed consolidated financial statements.
In November 2023, the FASB issued ASU No. 2023-07, Improvements to Reportable Segment Disclosures (Topic 280). This ASU updates reportable segment disclosure requirements by requiring disclosures of significant reportable segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a segment’s profit or loss. This ASU also requires disclosure of the title and position of the individual identified as the CODM and an explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and deciding how to allocate resources. The ASU is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. The Company adopted this ASU on January 1, 2024.
Management does not believe that any recently issued, but not yet effective, accounting standards could have a material effect on the accompanying financial statements. As new accounting pronouncements are issued, the Company will adopt those that are applicable under the circumstances.
NOTE 4: FAIR VALUE MEASUREMENTS
The Company’s financial assets and liabilities subject to fair value measurements on a recurring basis and the level of inputs used for such measurements were as follows:
|
Fair Value Measurements as of September 30, 2025 Using: |
||||||||||||
|
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||
|
Assets: |
|
|
|
|
||||||||
|
Digital assets, at fair value |
$ |
118,971,323 |
$ |
13,305,750 |
$ |
— |
$ |
132,277,073 |
||||
|
$ |
118,971,323 |
$ |
13,305,750 |
$ |
— |
$ |
132,277,073 |
|||||
|
Fair Value Measurements as of March 31, 2025 Using: |
||||||||||||
|
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||
|
Assets: |
|
|
|
|
||||||||
|
Digital assets, at fair value |
$ |
84,481,633 |
$ |
54,383,378 |
$ |
— |
$ |
138,865,011 |
||||
|
$ |
84,481,633 |
$ |
54,383,378 |
$ |
— |
$ |
138,865,011 |
|||||
Digital assets, at fair value
The Company measures its digital assets in accordance with the fair value hierarchy prescribed by ASC 820, Fair Value Measurement, which prioritizes the use of observable market inputs. Level 1 inputs consist of unadjusted quoted prices in active markets that the Company can access on the measurement date. An active market is one in which transactions occur with sufficient frequency and volume to provide ongoing pricing information. When an active market is not present, the Company uses observable inputs other than quoted prices (Level 2) or, when necessary, unobservable inputs (Level 3). Significant adjustments to Level 2 inputs result in classification within Level 3.
BTC is traded continuously on multiple Tier-1 global exchanges with significant daily trading volume, providing readily accessible quoted prices; therefore, BTC is classified as a Level 1 asset.
F-47
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 4: FAIR VALUE MEASUREMENTS (cont.)
NILA is a micro-cap token with limited trading volume and without listing on major U.S. exchanges, does not have an active market and is classified as a Level 2 asset.
Tether (USDT) is traded in an active market on the Company’s principal exchange, providing unadjusted quoted prices for identical assets; therefore, Tether (USDT) is classified as a Level 1 asset.
Lbank Wallet: In accordance with ASC 350-60 and ASC 820, the Company measures digital assets at fair value at each reporting date. As these assets are traded on an active exchange with unadjusted quoted prices, they are classified as Level 1 within the fair value hierarchy. All realized and unrealized gains or losses are recognized in the period of change within the statement of operations.
NOTE 5: PROPERTY AND EQUIPMENT
As of September 30, 2025 and March 31, 2025, property and equipment consist of:
|
September 30, |
March 31, |
||||||
|
Computer and equipment |
$ |
— |
$ |
1,416 |
|
||
|
Less: Accumulated depreciation |
|
— |
|
(590 |
) |
||
|
Property and equipment, net |
$ |
— |
$ |
826 |
|
||
Depreciation expense was $0 and $142 for the six months ended September 30, 2025 and 2024, respectively, which is included in general and administrative expenses in the consolidated
statements of operations.
During the six months ended September 30, 2025, the Company written off $826 of property and equipment net of accumulated depreciation.
NOTE 6: DIGITAL ASSETS, AT FAIR VALUE
The table below details, as of September 30, 2025, the components of digital assets at fair value with units, cost basis amounts and fair value.
|
September 30, 2025 |
||||||||
|
Units |
Cost Basis |
Fair Value |
||||||
|
Nila Tokens |
801,551,179 |
$ |
400,776 |
$ |
13,305,750 |
|||
|
BTC (Bitcoin) |
1,000 |
|
82,437,860 |
|
114,396,520 |
|||
|
Tether (USDT) Tokens |
4,571,503 |
|
4,571,503 |
|
4,571,503 |
|||
|
Lbank Wallet |
3,300 |
|
3,300 |
|
3,300 |
|||
|
806,126,982 |
$ |
87,413,439 |
$ |
132,277,073 |
||||
|
March 31, 2025 |
||||||||
|
Units |
Cost Basis |
Fair Value |
||||||
|
Nila Tokens |
906,389,631 |
$ |
453,194 |
$ |
54,383,378 |
|||
|
BTC (Bitcoin) |
1,000 |
|
82,437,860 |
|
82,437,860 |
|||
|
Tether (USDT) Tokens |
2,043,773 |
|
2,043,773 |
|
2,043,773 |
|||
|
908,434,404 |
$ |
84,934,827 |
$ |
138,865,011 |
||||
F-48
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 6: DIGITAL ASSETS, AT FAIR VALUE (cont.)
The table below shows a reconciliation of activity of the digital assets, at fair value.
|
Six Months Ended |
||||||||
|
2025 |
2024 |
|||||||
|
Beginning balance |
$ |
138,865,011 |
|
$ |
— |
|
||
|
Purchases |
|
— |
|
|
528,510 |
|
||
|
Sales |
|
(10,532,210 |
) |
|
(7,394,745 |
) |
||
|
Gross trading gains |
|
6,557,100 |
|
|
— |
|
||
|
Gross trading expenses |
|
(6,553,800 |
) |
|
— |
|
||
|
Realized gains |
|
10,479,790 |
|
|
7,333,122 |
|
||
|
Sale consideration received as digital assets (net of expenses) |
|
2,527,730 |
|
|
1,860,073 |
|
||
|
Unrealized losses |
|
(9,066,549 |
) |
|
56,026,550 |
|
||
|
Ending balance |
$ |
132,277,073 |
|
$ |
58,353,510 |
|
||
The cost price of the NILA, BTC and Tether tokens purchased was $0.0005 per share, $82,438 per share and $1.00 per share, respectively. The disposal price of the NILA token sales was $0.09 to 0.11 per share during the six months ended September 30, 2025. The disposal price of the NILA token sales was $0.06 per share during the six months ended September 30, 2024.
As of September 30, 2025, $16,802,676 is held with the joint wallet custody of the Chief Executive Officer along with the company. There is no restriction with the company with respect to the sale or transfer of any of such wallets.
NOTE 7: STOCKHOLDERS’ EQUITY
The Company’s certificate of incorporation authorized the Company to issue 1,500 shares of common stock with a par value of $0.01 per share.
Common Stock
On November 10, 2025, the Company entered into the Reorganization Agreement and Plan of Share Exchange, pursuant to which we issued to the shareholders 1,001 shares of our Common Stock in exchange for the 100% of membership interests of TechyTrade FZ-LLC owned by TechyTrade Singapore. As a result, the Company became the sole member of TechyTrade FZ-LLC and it became the wholly owned subsidiary with the shareholders the holders of 1,001 shares of our common stock.
As of September 30, 2025 and March 31, 2025, there were 1,001 and 1,001 shares of common stock issued and outstanding, respectively.
TechyTrade FZ-LLC Capital Transaction
During the year ended March 31, 2025, the Company completed a non-cash transaction in connection with the acquisition of digital assets. As consideration for the assets received, the Company issued equity shares with an aggregate fair value of $82,966,370, which was included in additional paid-in capital (see above for Reorganization of the capital structure). The fair value assigned to the ordinary shares was determined based on the negotiated transaction price agreed upon with the counterparty as of the issuance date.
F-49
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 8: RELATED PARTIES
During the six months ended September 30, 2024, the Company acquired NILA tokens from a related party entity (M/s Calfin Capital) with common control and management. The tokens were acquired at an arm’s length cost basis.
NOTE 9: SEGMENT REPORTING
The Chief Operating Decision Maker (CODM) has been identified as the Chief Executive Officer, who reviews the operating results for the Company as a whole to make decisions about allocating resources and assessing financial performance. Accordingly, management has determined that the Company only has one operating and reportable segment.
When evaluating the Company’s performance and making key decisions regarding resource allocation the CODM reviews several key metrics, which include the following:
|
Six Months Ended |
|||||||
|
2025 |
2024 |
||||||
|
Total operating expense (income) |
$ |
6,588,764 |
$ |
(57,824,858 |
) |
||
The key measures of segment profit or loss reviewed by our CODM are operating expenses/income. Operating costs are reviewed and monitored by the CODM to manage and forecast cash. The CODM also reviews operating costs to manage, maintain and enforce all contractual agreements to ensure costs are aligned with all agreements and budget.
NOTE 10: COMMITMENTS AND CONTIGENCIES
NILA Token Service Agreement
Effective April 2024, the Company entered into an exclusive Service Agreement with a Distributor and Service Provider for the sale, distribution and various marketing and administrative services regarding the Company’s NILA tokens.
The Company’s primary operational and financial commitment is the mandated payment structure, which requires the deduction and payment of 44% of gross token sale proceeds as commission to the Distributor and 32% (or a fixed fee) as service fees to the Service Provider. This structure represents a firm commitment to future cash outflows, contingent on the generation of token sales revenue. The Distributor is committed to exclusive sales and collection, and the Service Provider is committed to providing all marketing, legal, administrative, and security services for the token issuance.
The Service Agreement commences on the effective date and continues until the completion of the token sale, unless terminated earlier by mutual agreement or for cause.
Contingencies
The Company’s operations are subject to a variety of local and state regulations. Failure to comply with one or more of those regulations could result in fines, restrictions on its operations, or losses of permits that could result in the Company ceasing operations.
Litigation and Claims
From time to time, the Company may be involved in litigation relating to claims arising out of operations in the normal course of business. As of September 30, 2025, there were no pending or threatened lawsuits that could reasonably be expected to have a material effect on the results of the Company’s operations.
F-50
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 11: SUBSEQUENT EVENTS
Formation of MindWave Innovations Inc.
Mindwave Innovations Inc. was incorporated under the laws of the State of Delaware as on November 10, 2025 to become the ultimate holding company of TechyTrade FZ-LLC and, a company incorporated in United Arab Emirates and owned by TechyTrade Singapore.
On November 10, 2025, Mindwave Innovations Inc. entered into a Reorganization Agreement and Plan of Share Exchange to consolidate its corporate structure. Under this agreement, the Company issued 1,001 shares of its common stock in exchange for 100% of the membership interests in TechyTrade FZ-LLC, which was previously held by TechyTrade Singapore. As a result of this transaction, TechyTrade FZ-LLC became a wholly owned subsidiary of Mindwave Innovations Inc., establishing the Company as the ultimate holding entity for the group.
Merger Agreement with Apimeds Pharmaceuticals
On December 1, 2025 (the “Closing Date”), Apimeds Pharmaceuticals US, Inc., a Delaware corporation (“Apimeds” or “Acquiror”), entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Apimeds Merger Sub, Inc., a Delaware corporation (“Merger Sub”), MindWave Innovations Inc, a Delaware corporation (the “Company”), Lokahi Therapeutics, Inc., a Nevada corporation (“Bio Sub”), and Erik Emerson, solely in his capacity as representative for the Bio Business (the “Bio Business Representative”). The transactions contemplated by the Merger Agreement are referred to herein as the “Transactions” and the closing of the Transactions is referred to herein as the “Closing”. The Closing occurred simultaneously with the execution and delivery of the Merger Agreement on the Closing Date.
At the Effective Time, by virtue of the Merger and without any action on the part of the Company, Acquiror, Merger Sub or the holder of any existing common stock of the Company (the “Existing Company Common Stock”): (i) each share of common stock of Merger Sub, issued and outstanding immediately prior to the Effective Time was converted into one validly issued, fully paid and nonassessable share of common stock of the Company (the “Company Common Stock”); and (ii) each share of Existing Company Common Stock and existing preferred stock of the Company (collectively, the “Existing Company Stock”) issued and outstanding immediately prior to the Effective Time was canceled and converted into the right to receive a portion of the Merger Consideration (as defined herein), consisting of shares of Series A convertible preferred stock, par value $0.01 per share of the Acquiror (the “Acquiror Preferred Stock”), with each holder of such shares receiving, for each share of Existing Company Stock held immediately prior to the Effective Time, a pro rata portion of the Merger Consideration, a number of duly authorized, validly issued, fully paid and nonassessable shares of Acquiror Preferred Stock, such that, immediately following the Effective Time, the holders of Existing Company Stock collectively held, on an as-converted to Acquiror Common Stock basis 90.9% of the equity capital of the Acquiror as of the Closing Date. The shares of Acquiror Preferred Stock, and Company Common Stock issued in connection with the Business Combination are collectively referred to as the “Merger Consideration.”
Management has evaluated subsequent events through January 8, 2025, the date the financial statements were available to be issued. Based on this evaluation, no additional material events were identified which require adjustment or disclosure in these financial statements.
F-51
Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors of
MINDWAVE INNOVATIONS INC.
OPINION ON THE FINANCIAL STATEMENTS
We have audited the accompanying consolidated balance sheets of Mindwave Innovations Inc., (the “group”) as of March 31, 2025 and 2024, the related consolidated statements of operations and consolidated comprehensive income (profit), consolidated statements of member’s equity and consolidated statements of cash Flows for the year ended March 31, 2025, and March 31, 2024, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the consolidated financial position of the group as at March 31, 2025 and 2024, and the consolidated results of its operations and its consolidated cash flows for the year ended March 31, 2025, and March 31, 2024, in conformity with Generally Accepted Accounting Principles of United States of America.
BASIS FOR OPINION
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The company is not required to have nor we have engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Suri & Co., Chartered Accountants
We have served as the Company’s auditors since 2025.
Place: Mumbai, India
Date: January 4, 2026
F-52
CONSOLIDATED BALANCE SHEETS
|
March 31, |
|||||||
|
2025 |
2024 |
||||||
|
ASSETS |
|
|
|
||||
|
Current assets: |
|
|
|
||||
|
Cash and cash equivalents |
$ |
6 |
$ |
7 |
|
||
|
Total current assets |
|
6 |
|
7 |
|
||
|
Digital assets, at fair value |
|
138,865,011 |
|
— |
|
||
|
Property and equipment, net |
|
826 |
|
1,109 |
|
||
|
Total assets |
$ |
138,865,843 |
$ |
1,116 |
|
||
|
|
|
|
|||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
||||
|
Liabilities: |
$ |
— |
$ |
— |
|
||
|
|
|
|
|||||
|
Stockholders’ equity: |
|
|
|
||||
|
Common stock, 1,500 shares authorized, $0.01 par, 1,001 shares issued and outstanding as of both March 31, 2025 and 2024 |
|
10 |
|
10 |
|
||
|
Additional paid-in capital |
|
82,969,083 |
|
2,713 |
|
||
|
Retained earnings (deficit) |
|
55,896,750 |
|
(1,607 |
) |
||
|
Total stockholders’ equity |
|
138,865,843 |
|
1,116 |
|
||
|
Total liabilities and stockholders’ equity |
$ |
138,865,843 |
$ |
1,116 |
|
||
See accompanying notes to consolidated financial statements.
F-53
CONSOLIDATED STATEMENTS OF OPERATIONS
|
Year Ended March 31, |
|||||||
|
2025 |
2024 |
||||||
|
Revenue |
$ |
— |
|
$ |
621,169 |
||
|
Cost of revenue |
|
— |
|
|
83,895 |
||
|
Gross profit |
$ |
— |
|
$ |
537,274 |
||
|
|
|
|
|||||
|
Operating (income) expense: |
|
|
|
||||
|
General and administrative |
|
2,674,157 |
|
|
111,348 |
||
|
Distribution and commission expenses |
|
3,718,158 |
|
|
— |
||
|
Realized gain on sale of digital assets |
|
(8,360,488 |
) |
|
— |
||
|
Unrealized gain from changes in fair value of digital assets |
|
(53,930,184 |
) |
|
— |
||
|
Total operating (income) expense |
|
(55,898,357 |
) |
|
111,348 |
||
|
Income from operations |
|
55,898,357 |
|
|
425,927 |
||
|
|
|
|
|||||
|
Other income (expense): |
|
|
|
||||
|
Forgiveness of debt |
|
— |
|
|
32,007 |
||
|
Total other income (expense), net |
|
— |
|
|
32,007 |
||
|
|
|
|
|||||
|
Provision for income taxes |
|
— |
|
|
— |
||
|
Net income |
$ |
55,898,357 |
|
$ |
457,933 |
||
|
|
|
|
|||||
|
Net income per share – basic and diluted |
$ |
55,842.51 |
|
$ |
457.48 |
||
|
Weighted average common shares outstanding – basic and diluted |
|
1,001 |
|
|
1,001 |
||
See accompanying notes to consolidated financial statements.
F-54
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
Additional |
Accumulated |
Total |
|||||||||||||
|
Number |
Amount |
|||||||||||||||
|
Balances at March 31, 2023 |
1,001 |
$ |
10 |
$ |
2,713 |
$ |
(459,540 |
) |
$ |
(456,817 |
) |
|||||
|
Net income |
— |
|
— |
|
— |
|
457,933 |
|
|
457,933 |
|
|||||
|
Balances at March 31, 2024 |
1,001 |
|
10 |
|
2,713 |
|
(1,607 |
) |
|
1,116 |
|
|||||
|
Issuance of equity shares (see Note 7) |
— |
|
— |
|
82,966,370 |
|
— |
|
|
82,966,370 |
|
|||||
|
Net income |
— |
|
— |
|
— |
|
55,898,357 |
|
|
55,898,357 |
|
|||||
|
Balances at March 31, 2025 |
1,001 |
$ |
10 |
$ |
82,969,083 |
$ |
55,896,750 |
|
$ |
138,865,843 |
|
|||||
See accompanying notes to consolidated financial statements.
F-55
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
Year Ended March 31, |
||||||||
|
Cash flows from operating activities: |
2025 |
2024 |
||||||
|
Net income |
$ |
55,898,357 |
|
$ |
457,933 |
|
||
|
Adjustments to reconcile net income to net cash (used in) provided by operating activities: |
|
|
|
|
||||
|
Realized gain from sale of digital assets |
|
(8,360,488 |
) |
|
— |
|
||
|
Unrealized gain from changes in fair value of digital assets |
|
(53,930,184 |
) |
|
— |
|
||
|
Forgiveness of debt |
|
— |
|
|
(32,007 |
) |
||
|
Depreciation |
|
283 |
|
|
283 |
|
||
|
Changes in operating assets and liabilities: |
|
|
|
|
||||
|
Accounts receivable |
|
— |
|
|
4,520 |
|
||
|
Accrued expenses |
|
— |
|
|
(7,951 |
) |
||
|
Net cash (used in) provided by operating activities |
|
(6,392,032 |
) |
|
422,779 |
|
||
|
|
|
|
|
|||||
|
Cash flows from investing activities: |
|
|
|
|
||||
|
Purchase of digital assets |
|
(2,043,773 |
) |
|
— |
|
||
|
Sale of digital assets |
|
8,435,804 |
|
|
— |
|
||
|
Net cash provided by investing activities |
|
6,392,031 |
|
|
— |
|
||
|
|
|
|
|
|||||
|
Cash flows from financing activities: |
|
|
|
|
||||
|
Repayment of loan – related parties |
|
— |
|
|
(423,254 |
) |
||
|
Net cash used in financing activities |
|
— |
|
|
(423,254 |
) |
||
|
Net change in cash |
|
(1 |
) |
|
(475 |
) |
||
|
Cash at beginning of year |
|
7 |
|
|
482 |
|
||
|
Cash at end of year |
$ |
6 |
|
$ |
7 |
|
||
|
|
|
|
|
|||||
|
Supplemental disclosure of cash flow information: |
|
|
|
|
||||
|
Cash paid for interest |
$ |
— |
|
$ |
— |
|
||
|
Cash paid for taxes |
$ |
— |
|
$ |
— |
|
||
|
|
|
|
|
|||||
|
Supplemental disclosure to investing and financing activities: |
|
|
|
|
||||
|
Non-cash purchase of digital assets in exchange of equity shares issued |
$ |
82,966,370 |
|
$ |
— |
|
||
See accompanying notes to consolidated financial statements.
F-56
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1: NATURE OF OPERATIONS
Mindwave Innovations Inc. (the “Company”) was incorporated under the laws of the State of Delaware on November 10, 2025 to become the ultimate holding company of TechyTrade FZ-LLC, a company incorporated in United Arab Emirates, and TechyTrade Innovations Pte. Ltd, a company incorporated in Singapore. TechyTrade Innovations Pte. Ltd. (“TechyTrade Singapore”) was incorporated in Singapore on September 8, 2025. TechyTrade FZ-LLC was originally a company incorporated in United Arab Emirates on December 27, 2020 and is the entity that maintains historical operations of the Company.
The Company is a leading provider of institutional Digital Asset Treasury (DAT) solutions, specializing in compliant Bitcoin treasury infrastructure, AI-driven yield capabilities, ClimateTech impact systems, and AdTech engagement platforms. The company’s multi-vertical ecosystem is powered by its native token, $NILA, which enables governance, utility, and value flow across its blockchain-integrated operations.
MindWave is a global leader in AI-driven Bitcoin and yield-generation technologies, operating in one of the fastest-growing segments of the digital-asset market. Bitcoin remains the most established and highly valued cryptocurrency, and MindWave’s platform is designed to help institutions securely hold, manage, and generate yield from Bitcoin reserves.
MindWave’s three-pronged strategic framework includes:
1. Secure Digital Treasury Wallets for Corporations,
2. AI-Enhanced Bitcoin Yield Generation, and
3. A Validator-Powered Ecosystem supported by the $NILA Token. Together, these capabilities make
MindWave one of the first companies to pursue a publicly traded, institutional-focused Digital Asset Treasury (DAT) model.
Reorganization
Prior to the reorganization transaction described below, TechyTrade FZ-LLC maintained historical operarations of the Company since its formation in 2020. In September 2025, TechyTrade FZ-LLC ebcame a wholly owned subsidiary of TechyTrade Innovations Pte. Ltd.
On November 10, 2025, Mindwave Innovations Inc. entered into a Reorganization Agreement and Plan of Share Exchange to consolidate its corporate structure. Under this agreement, the Company issued 1,001 shares of its common stock in exchange for 100% of the membership interests in TechyTrade FZ-LLC, which was previously held by TechyTrade Singapore. As a result of this transaction, TechyTrade FZ-LLC became a wholly owned subsidiary of Mindwave Innovations Inc., establishing the Company as the ultimate holding entity for the group.
The Reorganization is being accounted for as a reorganization of entities under common control. The accompanying financial statements have been presented to retroactively present the effect of the Reorganization. See Note 3 for further detail.
NOTE 2: LIQUIDITY AND CASH RESOURCES
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business.
The Company’s primary sources of liquidity have been cash flows from operating in sale of digital tokens. For the year ended March 31, 2025, the Company reported income from operations of $55,898,357. The company has no liability to be settled to the outsiders.
F-57
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 2: LIQUIDITY AND CASH RESOURCES (cont.)
Management has evaluated the Company’s ability to continue as a going concern within one year after the date the financial statements are issued, as required under ASC 205-40. Based on this evaluation, management has concluded that there are no conditions or events that raise substantial doubt about the Company’s ability to continue as a going concern. Accordingly, no material uncertainties have been identified that would cast significant doubt on the Company’s ability to meet its obligations as they become due.
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America (“US GAAP”).
The Company’s fiscal year is March 31.
The financial statements included herein comprise the consolidated results of the Company and its subsidiaries. All intercompany transactions have been eliminated. The consolidated financial statements represent the historical operations of TechyTrade FZ-LLC, which was incorporated in United Arab Emirates and the initial capital structure of Mindwave Innovations Inc., which was completed upon the Reorganization (see Note 1).
In accordance with ASC 805-50-15-6, the Company determined that the share exchange was a reorganization of entities under common control. Mindwave Innovations Inc. and TechyTrade FZ-LLC maintained common control for the entire period for which the financial statements are presented through the Reorganization. The Company concluded that the entities were under common control via common ownership and common management. Therefore, in accordance with ASC 250-10-45 and ASC 805-50-45, the financial statements require retrospective consolidation of the entities for all periods presented. The financial statements for the years ended March 31, 2025 and 2024 are prepared on a consolidated basis of the Company and TechyTrade FZ-LLC.
The consolidated financial statements as of and for the years ended March 31, 2025 and 2024 have been presented to reflect the historical operations of the Company for the periods presented. The financial statements reflect the revenues, expenses, assets, and liabilities to reflect all historical results of operations of the business.
Use of Estimates
The preparation of the accompanying financial statements in conformity with GAAP requires management to make certain estimates and assumptions that affect the reported amounts and disclosures of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. These estimates are based on information available through the date of the issuance of the financial statements and actual results could differ from those estimates. Estimates are adjusted to reflect actual experience when necessary. Significant estimates made by management include, but are not limited to:
• revenue recognition
• credit loss on accounts receivables and contract receivables
• provisions for income taxes and related valuation allowances and tax uncertainties
• recoverability of long-lived assets and their related estimated lives
• impairment of crypto currencies
• accruals for estimated liabilities
F-58
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Cash and Cash Equivalents
Cash and cash equivalents include all cash in banks. The Company considers all highly liquid instruments with original maturities of three months or less to be cash equivalents.
Concentration of Credit Risk
The Fund maintains its cash with a bank located in the United Arab Emirates and believes it to be creditworthy. Balances are covered by the local deposit insurance regulations as established by the Central Bank of the UAE. At times, the Fund may maintain balances in excess of these regulatory coverage limits.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation and impairments. Normal repairs and maintenance costs are charged to earnings as incurred and additions and major improvements are capitalized. The cost of assets retired or otherwise disposed of, and the related depreciation are eliminated from the accounts in the period of disposal and the resulting gain or loss is credited or charged to earnings.
Depreciation is computed over the estimated useful lives of the related asset type using the straight-line method for financial statement purposes. The estimated useful lives for property and equipment consist of computer and equipment and straight-line depreciation is based on a useful life of 5 years.
Fair Value of Financial Instruments
We determine fair value measurements for certain assets and liabilities in accordance with Accounting Standards Codification Topic 820, Fair Value Measurements (“ASC 820”), which defines fair value as the exit price that would be received to sell an asset or paid to transfer a liability in an ordinary transaction between market participants. ASC 820 establishes a framework for valuation techniques, prioritized by reliability, according to the following tiers:
|
Level 1 — |
Unadjusted quoted prices in active markets for identical assets and liabilities |
|||
|
Level 2 — |
Quoted prices for similar assets and liabilities in active markets; quoted prices for similar or identical assets and liabilities in markets that are not active; valuation models in which all significant inputs are derived from observable market data |
|||
|
Level 3 — |
Unobservable valuation model inputs for assets and liabilities such as discounted cash flow models or similar techniques; inputs for fair value instruments; includes assumptions and may require significant judgment and estimation by management |
We use this framework to measure the fair value of certain financial instruments on a recurring basis, such as our cash and cash equivalents and digital assets at fair value, as well as on a non-recurring basis for our acquisitions and impairment testing on our property and equipment. See Note 4 — Fair Value Measurements for further discussion.
Net Income per Share
Net earnings per share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period, excluding shares subject to redemption or forfeiture. The Company presents basic and diluted net earnings or loss per share. Diluted net earnings or loss per share reflect the actual weighted average of common shares issued and outstanding during the period, adjusted for potentially dilutive securities outstanding. Potentially dilutive securities are excluded from the computation of the diluted net income per share if their inclusion would be anti-dilutive. As of March 31, 2025 and 2024, there were no potentially dilutive shares outstanding.
F-59
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Digital Assets
Digital assets are considered intangible assets under Accounting Standards Codification Subtopic 350-60, Intangibles — Goodwill and Other — Crypto Assets (“ASC 350-60”) and Accounting Standards Update 2023-08. Under ASC 350-60, digital assets that meet the definition of intangible assets, among other criteria, are initially recorded at cost and are then subsequently remeasured at fair value, as of the balance sheet date with changes from remeasurement recognized in net income. The fair value for digital assets that are unrestricted (“liquid”) and freely tradable are determined using Level 1 inputs as defined in ASC 820. Conversely, fair value for digital assets that are restricted (“locked”) due to vesting schedules or contractual restrictions are determined based on Level 1 inputs adjusted for the impact of such restrictions based on Level 2 inputs, in accordance with ASC 820. We account for changes from remeasurement within (gain) loss from changes in fair value of digital assets, as stated on our statements of operations.
Digital assets that we hold that do not meet the criteria under ASC 350-60 are accounted for as intangible assets with indefinite useful lives and are initially recorded at cost and are not amortized but instead are tested for impairment at least annually, or more frequently, if events or changes in circumstances indicate that it is more likely than not that the asset is impaired. As such, these digital assets are reflected in our balance sheets, at cost net of any impairments within Digital assets, at carrying value. We evaluate impairments on these digital assets on at least an annual basis, or more frequently if events or changes in circumstances occur and use Level 1 inputs, unadjusted quoted prices in active markets, for the fair value to determine if an impairment has occurred. If unadjusted quoted prices in active markets for our digital assets are lower than the carrying value recorded, then we will record an impairment charge equal to the difference between the carrying value and the fair value. Impairment charges are recognized within (Gain) loss on changes in fair value of digital assets, as stated on our statements of operations.
When we receive digital assets earned on third party staking platforms, we will initially record them at fair value on the date the digital assets are received. Upon the disposal of digital assets, any gains or losses are recognized in realized gain on sale of digital assets, as stated on our statements of operations, and are calculated as the difference between the selling price and our carrying value, using the specific identification method. See Note 6 — Digital Assets for further discussion.
Impairment of Long-lived Assets
Long-lived assets, such as property, equipment, and identifiable intangibles with finite useful lives, are periodically evaluated for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company looks for indicators of a trigger event for asset impairment and pays special attention to any adverse change in the extent or manner in which the asset is being used or in its physical condition. Assets are reviewed using factors including, but not limited to, future operating plans and projected cash flows. The determination of whether impairment has occurred is based on an estimate of undiscounted future cash flows directly related to the assets, compared to the carrying value of the assets. If the sum of the undiscounted future cash flows of the assets does not exceed the carrying value of the assets, full or partial impairment may exist. If the asset carrying amount exceeds its fair value, an impairment charge is recognized in the amount by which the carrying amount exceeds the fair value of the asset. Fair value is determined using an income approach, which requires discounting the estimated future cash flows associated with the asset.
As of March 31, 2025 and 2024, there was no impairment for long-lived assets.
Revenue Recognition
We recognize revenue in accordance with the five-step model outlined in Accounting Standard Codification Topic 606 — Revenue from Contracts with Customers (“ASC 606”). This model helps us identify the contract we have with our customers, identify the performance obligation in the contract, determine the transaction price, allocate the transaction price to the performance obligation and recognize revenue as the performance obligation is satisfied. We earn revenue from consulting services to customers.
F-60
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
During the year ended March 31, 2025, the Company has not generated any revenue form from consulting services. The Company had realized and unrealized gains (losses) related to digital assets (see Note 6).
During the year ended March 31, 2024, the Company generated $621,169 in revenue from consulting services. The revenue was recognized at the point in time when the services were completed, upon when the performance obligations were fulfilled.
All revenue activity, and digital asset activity, were originated in the United Arab Emirates.
Cost of Revenue
Cost of revenue includes direct costs and costs related to marketing and technical consultancy.
Contract Balances
The Company does not typically record contract assets or contract liabilities, as consideration for token sales is received at or near the time control is transferred. No material contract asset or deferred revenue balances existed as of the reporting date unless otherwise disclosed.
Market Risk
The Company is exposed to market risk related to the digital asset holdings, which are impacted by the market value of the respective underlying digital asset. The Company performed a sensitivity analysis assuming a hypothetical 10% change in the fair value of these digital assets to demonstrate the potential impact on the financial results. A hypothetical 10% increase or decrease in market prices would have positively or negatively impacted Income (loss) before income taxes by approximately $13.9 million for the year ended March 31, 2025.
General and Administrative
General and administrative expenses primarily consist of administrative costs and marketing and promotional expenses related to digital assets. Pursuant to a service agreement (see Note 10), the Company receives legal, administrative support, compliance (KYC/AML), reporting and security services for the underlying NILA tokens. These costs are expensed as incurred.
Distribution and Commission
Distribution and commission expenses primarily consist of deduction and payment of 44% of gross token sale proceeds as commission to the Distributor. Pursuant to a service agreement (see Note 10), the Distributor is committed to exclusive sales and collection of NILA tokens. These costs are expensed as incurred.
Concentrations
In 2025, nearly all of the Company’s general and administrative and sales and marketing expenses were incurred to pursuant to one service agreement (see Note 10). The Company is dependent on two third-party vendors for its NILA token digital asset services and operations. The termination of this service agreement may have a negative short-term impact on the Company’s operations; however, the Company believes there are acceptable substitute vendors that can be utilized longer-term.
Segment Reporting
ASC Topic 280, “Segment Reporting,” establishes standards for companies to report in their financial statement information about operating segments, products, services, geographic areas, and major customers. Operating segments are defined as components of an enterprise for which separate financial information is available that is regularly evaluated by the Company’s chief operating decision maker, or group, in deciding how to allocate resources and assess performance.
F-61
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Foreign Currency Translation
For the years ended March 31, 2025 and 2024, the functional and reporting currency is the U.S. Dollar (“USD”).
Related Parties
Related parties are any entities or individuals that, through employment, ownership or other means, possess the ability to direct or cause the direction of the management and policies of the Company. The Company discloses related party transactions that are outside of normal compensatory agreements, such as loans. The Company follows ASC 850, Related Party Disclosures, for the identification of related parties and disclosure of related party transactions.
Income Taxes
The Fund is organized as a Limited Partnership (LP) and is treated as a pass-through entity for U.S. federal income tax purposes. Accordingly, the Fund itself is generally not subject to U.S. federal income tax on its income; instead, the Fund’s taxable income or loss is allocated to the Partners who are responsible for their own tax obligations. The Fund may be subject to income or excise taxes in certain state and local jurisdictions. The Fund was historically a juridical entity in the UAE. Following the introduction of the UAE Corporate Tax (CT) regime, the Fund has determined that it meets the requirements to be treated as a Qualifying Investment Fund (QIF) or similar exempt status, and is therefore not subject to the UAE CT on its core investment income. Management has evaluated the Fund’s tax positions and determined there are no material uncertain tax positions requiring recognition as of the reporting date.
Recently Issued and Adopted Accounting Pronouncements
In July 2025, Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2025-05 Financial Instruments — Credit Losses (“ASU 2025-05”), which provides a practical expedient that can be elected to be applied to accounts receivable and contract assets, which would allow entities to assume that current conditions as of the balance sheet date do not change for the remaining life of the assets when estimating expected credit losses for such assets. ASU 2025-05 applies to public entities with annual reporting periods beginning after December 15, 2025, and interim reporting periods within those annual reporting periods. Early adoption is permitted in both interim and annual reporting periods in which financial statements have not yet been issued or made available for issuance. We are evaluating the impact this guidance may have on our financial statements.
In November 2024, FASB issued ASU No. 2024-03, Income Statement — Reporting Comprehensive Income — Expense Disaggregation Disclosures (“ASU 2024-03”), which enhances transparency by requiring public entities to disclose more detailed information about their income statement expenses. This includes disaggregating specific natural expense categories, like employee compensation and depreciation, within certain expense captions. ASU 2024-03 applies to public entities with annual reporting periods beginning after December 15, 2026, and interim reporting periods beginning after December 15, 2027, early adoption is permitted. We are evaluating the impact this amended guidance may have on the notes to our condensed consolidated financial statements.
In November 2023, the FASB issued ASU No. 2023-07, Improvements to Reportable Segment Disclosures (Topic 280). This ASU updates reportable segment disclosure requirements by requiring disclosures of significant reportable segment expenses that are regularly provided to the Chief Operating Decision Maker (“CODM”) and included within each reported measure of a segment’s profit or loss. This ASU also requires disclosure of the title and position of the individual identified as the CODM and an explanation of how the CODM uses the reported measures of a segment’s profit or loss in assessing segment performance and deciding how to allocate resources. The ASU is effective for annual periods beginning after December 15, 2023, and interim periods within fiscal years beginning after December 15, 2024. The Company adopted this ASU on January 1, 2024.
Management does not believe that any recently issued, but not yet effective, accounting standards could have a material effect on the accompanying financial statements. As new accounting pronouncements are issued, the Company will adopt those that are applicable under the circumstances.
F-62
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4: FAIR VALUE MEASUREMENTS
The Company’s financial assets and liabilities subject to fair value measurements on a recurring basis and the level of inputs used for such measurements were as follows:
|
Fair Value Measurements as of March 31, 2025 Using: |
||||||||||||
|
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||
|
Assets: |
|
|
|
|
||||||||
|
Digital assets, at fair value |
$ |
84,481,633 |
$ |
54,383,378 |
$ |
— |
$ |
138,865,011 |
||||
|
$ |
84,481,633 |
$ |
54,383,378 |
$ |
— |
$ |
138,865,011 |
|||||
|
Fair Value Measurements as of March 31, 2024 Using: |
||||||||||||
|
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||
|
Assets: |
|
|
|
|
||||||||
|
Digital assets, at fair value |
$ |
— |
$ |
— |
$ |
— |
$ |
— |
||||
|
$ |
— |
$ |
— |
$ |
— |
$ |
— |
|||||
Digital assets, at fair value
The Company measures its digital assets in accordance with the fair value hierarchy prescribed by ASC 820, Fair Value Measurement, which prioritizes the use of observable market inputs. Level 1 inputs consist of unadjusted quoted prices in active markets that the Company can access on the measurement date. An active market is one in which transactions occur with sufficient frequency and volume to provide ongoing pricing information. When an active market is not present, the Company uses observable inputs other than quoted prices (Level 2) or, when necessary, unobservable inputs (Level 3). Significant adjustments to Level 2 inputs result in classification within Level 3.
BTC is traded continuously on multiple Tier-1 global exchanges with significant daily trading volume, providing readily accessible quoted prices; therefore, BTC is classified as a Level 1 asset.
NILA, a micro-cap token with limited trading volume and without listing on major U.S. exchanges, does not have an active market and is classified as a Level 2 asset.
Tether (USDT) is traded in an active market on the Company’s principal exchange, providing unadjusted quoted prices for identical assets; therefore, Tether (USDT) is classified as a Level 1 asset.
NOTE 5: PROPERTY AND EQUIPMENT
As of March 31, 2025 and 2024, property and equipment consist of:
|
March 31, |
||||||||
|
2025 |
2024 |
|||||||
|
Computer and equipment |
$ |
1,416 |
|
$ |
1,416 |
|
||
|
Less: Accumulated depreciation |
|
(590 |
) |
|
(307 |
) |
||
|
Property and equipment, net |
$ |
826 |
|
$ |
1,109 |
|
||
Depreciation expense was $283 and $283 for the years ended March 31, 2025 and 2024, respectively, which is included in general and administrative expenses in the statements of operations.
F-63
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6: DIGITAL ASSETS, AT FAIR VALUE
The table below details, as of March 31, 2025, the components of digital assets at fair value with units, cost basis amounts and fair value. The Company did not hold any digital assets, at fair value as of March 31, 2024.
|
March 31, 2025 |
||||||||
|
Units |
Cost Basis |
Fair Value |
||||||
|
NILA Tokens |
906,389,631 |
$ |
453,194 |
$ |
54,383,378 |
|||
|
BTC (Bitcoin) |
1,000 |
|
82,437,860 |
|
82,437,860 |
|||
|
Tether (USDT) Tokens |
2,043,773 |
|
2,043,773 |
|
2,043,773 |
|||
|
908,434,404 |
$ |
84,934,827 |
$ |
138,865,011 |
||||
The table below shows a reconciliation of activity of the digital assets, at fair value.
|
Year Ended March 31, |
|||||||
|
2025 |
2024 |
||||||
|
Beginning balance |
$ |
— |
|
$ |
— |
||
|
Purchases |
|
82,966,370 |
|
|
— |
||
|
Sales |
|
(8,430,744 |
) |
|
— |
||
|
Realized gains |
|
8,360,488 |
|
|
— |
||
|
Promotional sales |
|
(5,060 |
) |
|
|||
|
Sale consideration received as digital assets (net of expenses) |
|
2,043,773 |
|
|
|||
|
Unrealized gains |
|
53,930,184 |
|
|
— |
||
|
Ending balance |
$ |
138,865,011 |
|
$ |
— |
||
The cost price of the NILA, BTC and Tether tokens purchased was $0.0005 per share, $82,438 per share and $1.00 per share, respectively. The disposal price of the NILA token sales was $0.06 per share.
As of March 31, 2025, $16,802,676 of the digital assets held by the Company is in joint wallet custody of the Chief Executive Officer. There is no restriction with the Company with respect to the sale or transfer of any of such wallets.
The Company held the 1,000 BTC as identifiable assets under its control, maintained in a segregated sub-wallet administered by MindWave, for the benefit of TechyTrade. The private keys, full beneficial ownership and control of the segregated wallets are held by and shall remain under the custody and ownership of TechyTrade FZ-LLC. Subsequent to March 31, 2025, the wallet was transferred to the custody of TechyTrade FZ LLC.
NOTE 7: STOCKHOLDERS’ EQUITY
The Company’s certificate of incorporation authorized the Company to issue 1,500 shares of common stock with a par value of $0.01 per share.
Common Stock
On November 10, 2025, the Company entered into the Reorganization Agreement and Plan of Share Exchange, pursuant to which we issued to the shareholders 1,001 shares of our Common Stock in exchange for the 100% of membership interests of TechyTrade FZ-LLC owned by TechyTrade Singapore. As a result, the Company became the sole member of TechyTrade FZ-LLC and it became the wholly owned subsidiary with the shareholders the holders of 1,001 shares of our common stock.
As of March 31, 2025 and 2024, there were 1,001 and 1,001 shares of common stock issued and outstanding, respectively.
F-64
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7: STOCKHOLDERS’ EQUITY (cont.)
TechyTrade FZ-LLC Capital Transaction
During the year ended March 31, 2025, the Company completed a non-cash transaction in connection with the acquisition of digital assets. As consideration for the assets received, the Company issued equity shares with an aggregate fair value of $82,966,370, which was included in additional paid-in capital (see above for Reorganization of the capital structure). The fair value assigned to the ordinary shares was determined based on the negotiated transaction price agreed upon with the counterparty as of the issuance date.
NOTE 8: RELATED PARTIES
During the year ended March 31, 2025, the Company acquired NILA tokens from a related party entity with common control and management. The tokens were acquired at an arm’s length cost basis.
During the year ended March 31, 2024, a related party loan with an outstanding balance of $455,261 was settled in full. This settlement was comprised of a debt forgiveness of $32,007 and a total cash repayment of $423,254. The gain on forgiveness of debt has been recorded within other income in the statements of operations.
NOTE 9: SEGMENT REPORTING
The Chief Operating Decision Maker (CODM) has been identified as the Chief Executive Officer, who reviews the operating results for the Company as a whole to make decisions about allocating resources and assessing financial performance. Accordingly, management has determined that the Company only has one operating and reportable segment.
When evaluating the Company’s performance and making key decisions regarding resource allocation the CODM reviews several key metrics, which include the following:
|
Year Ended March 31, |
|||||||
|
2025 |
2024 |
||||||
|
Total operating (income) expense |
$ |
(55,898,357 |
) |
$ |
111,348 |
||
The key measures of segment profit or loss reviewed by our CODM are operating expenses/income. Operating costs are reviewed and monitored by the CODM to manage and forecast cash. The CODM also reviews operating costs to manage, maintain and enforce all contractual agreements to ensure costs are aligned with all agreements and budget.
NOTE 10: COMMITMENTS AND CONTIGENCIES
NILA Token Service Agreement
Effective April 2024, the Company entered into an exclusive Service Agreement with a Distributor and Service Provider for the sale, distribution and various marketing and administrative services regarding the Company’s NILA tokens.
The Company’s primary operational and financial commitment is the mandated payment structure, which requires the deduction and payment of 44% of gross token sale proceeds as commission to the Distributor and 32% (or a fixed fee) as service fees to the Service Provider. This structure represents a firm commitment to future cash outflows, contingent on the generation of token sales revenue. The Distributor is committed to exclusive sales and collection, and the Service Provider is committed to providing all marketing, legal, administrative, and security services for the token issuance.
The Service Agreement commences on the effective date and continues until the completion of the token sale, unless terminated earlier by mutual agreement or for cause.
F-65
MINDWAVE INNOVATIONS INC.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10: COMMITMENTS AND CONTIGENCIES (cont.)
Contingencies
The Company’s operations are subject to a variety of local and state regulations. Failure to comply with one or more of those regulations could result in fines, restrictions on its operations, or losses of permits that could result in the Company ceasing operations.
Litigation and Claims
From time to time, the Company may be involved in litigation relating to claims arising out of operations in the normal course of business. As of March 31, 2025, there were no pending or threatened lawsuits that could reasonably be expected to have a material effect on the results of the Company’s operations.
NOTE 11: SUBSEQUENT EVENTS
Formation of MindWave Innovations Inc.
Mindwave Innovations Inc. was incorporated under the laws of the State of Delaware as on November 10, 2025 to become the ultimate holding company of TechyTrade FZ-LLC and, a company incorporated in United Arab Emirates and owned by TechyTrade Singapore.
On November 10, 2025, Mindwave Innovations Inc. entered into a Reorganization Agreement and Plan of Share Exchange to consolidate its corporate structure. Under this agreement, the Company issued 1,001 shares of its common stock in exchange for 100% of the membership interests in TechyTrade FZ-LLC, which was previously held by TechyTrade Singapore. As a result of this transaction, TechyTrade FZ-LLC became a wholly owned subsidiary of Mindwave Innovations Inc., establishing the Company as the ultimate holding entity for the group.
Merger Agreement with Apimeds Pharmaceuticals
On December 1, 2025 (the “Closing Date”), Apimeds Pharmaceuticals US, Inc., a Delaware corporation (“Apimeds” or “Acquiror”), entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Apimeds Merger Sub, Inc., a Delaware corporation (“Merger Sub”), MindWave Innovations Inc, a Delaware corporation (the “Company”), Lokahi Therapeutics, Inc., a Nevada corporation (“Bio Sub”), and Erik Emerson, solely in his capacity as representative for the Bio Business (the “Bio Business Representative”). The transactions contemplated by the Merger Agreement are referred to herein as the “Transactions” and the closing of the Transactions is referred to herein as the “Closing”. The Closing occurred simultaneously with the execution and delivery of the Merger Agreement on the Closing Date.
At the Effective Time, by virtue of the Merger and without any action on the part of the Company, Acquiror, Merger Sub or the holder of any existing common stock of the Company (the “Existing Company Common Stock”): (i) each share of common stock of Merger Sub, issued and outstanding immediately prior to the Effective Time was converted into one validly issued, fully paid and nonassessable share of common stock of the Company (the “Company Common Stock”); and (ii) each share of Existing Company Common Stock and existing preferred stock of the Company (collectively, the “Existing Company Stock”) issued and outstanding immediately prior to the Effective Time was canceled and converted into the right to receive a portion of the Merger Consideration (as defined herein), consisting of shares of Series A convertible preferred stock, par value $0.01 per share of the Acquiror (the “Acquiror Preferred Stock”), with each holder of such shares receiving, for each share of Existing Company Stock held immediately prior to the Effective Time, a pro rata portion of the Merger Consideration, a number of duly authorized, validly issued, fully paid and nonassessable shares of Acquiror Preferred Stock, such that, immediately following the Effective Time, the holders of Existing Company Stock collectively held, on an as-converted to Acquiror Common Stock basis 90.9% of the equity capital of the Acquiror as of the Closing Date. The shares of Acquiror Preferred Stock, and Company Common Stock issued in connection with the Business Combination are collectively referred to as the “Merger Consideration.”
Management has evaluated subsequent events through January 3, 2025, the date the financial statements were available to be issued. Based on this evaluation, no additional material events were identified which require adjustment or disclosure in these financial statements.
F-66
AGREEMENT AND PLAN OF MERGER
dated as of
December 1, 2025
by and among
APIMEDS PHARMACEUTICALS US, INC.,
as the Acquiror
APIMEDS MERGER SUB, INC.,
as the Merger Sub
MINDWAVE INNOVATIONS INC,
as the Company,
LOKAHI THERAPEUTICS, INC.,
as Bio Sub,
and
ERIK EMERSON
as the Bio Business Representative
TABLE OF CONTENTS
|
Page |
||||||
|
Article I CERTAIN DEFINITIONS |
A-1 |
|||||
|
Section 1.01 |
Definitions |
A-1 |
||||
|
Section 1.02 |
Construction |
A-9 |
||||
|
Article II THE MERGER; CLOSING |
A-10 |
|||||
|
Section 2.01 |
The Merger |
A-10 |
||||
|
Section 2.02 |
Effects of the Merger |
A-10 |
||||
|
Section 2.03 |
Closing |
A-10 |
||||
|
Section 2.04 |
Organizational Documents of the Company and Acquiror |
A-10 |
||||
|
Section 2.05 |
Directors and Officers of the Companies |
A-11 |
||||
|
Article III Effect on SECURITIES |
A-11 |
|||||
|
Section 3.01 |
Effect on Securities |
A-11 |
||||
|
Section 3.02 |
Withholding |
A-11 |
||||
|
Section 3.03 |
No Fractional Shares |
A-12 |
||||
|
Section 3.04 |
Reverse Stock Split |
A-12 |
||||
|
Section 3.05 |
Conversion of Preferred Stock |
A-12 |
||||
|
Article IV REPRESENTATIONS AND WARRANTIES OF THE COMPANY |
A-12 |
|||||
|
Section 4.01 |
Organization, Standing and Corporate Power |
A-12 |
||||
|
Section 4.02 |
Corporate Authority; Approval; Non-Contravention |
A-12 |
||||
|
Section 4.03 |
Governmental Approvals |
A-13 |
||||
|
Section 4.04 |
Capitalization |
A-13 |
||||
|
Section 4.05 |
Financial Statements; Internal Controls |
A-13 |
||||
|
Section 4.06 |
Compliance with Laws |
A-14 |
||||
|
Section 4.07 |
Absence of Certain Changes or Events |
A-14 |
||||
|
Section 4.08 |
No Undisclosed Liabilities |
A-14 |
||||
|
Section 4.09 |
Information Supplied |
A-14 |
||||
|
Section 4.10 |
Litigation |
A-14 |
||||
|
Section 4.11 |
Contracts |
A-15 |
||||
|
Section 4.12 |
Employee Benefits |
A-16 |
||||
|
Section 4.13 |
Labor and Employment |
A-17 |
||||
|
Section 4.14 |
Taxes |
A-18 |
||||
|
Section 4.15 |
Intellectual Property |
A-19 |
||||
|
Section 4.16 |
Data Protection |
A-20 |
||||
|
Section 4.17 |
Information Technology |
A-21 |
||||
|
Section 4.18 |
Real Property |
A-21 |
||||
|
Section 4.19 |
Anti-Bribery; Trade Controls Compliance |
A-22 |
||||
|
Section 4.20 |
Insurance |
A-22 |
||||
|
Section 4.21 |
Competition Regulation |
A-23 |
||||
|
Section 4.22 |
Environmental Matters |
A-23 |
||||
|
Section 4.23 |
Customers and Suppliers |
A-23 |
||||
|
Section 4.24 |
Brokers |
A-23 |
||||
|
Section 4.25 |
Affiliate Agreements |
A-23 |
||||
|
Section 4.26 |
Assets |
A-24 |
||||
|
Section 4.27 |
No Other Representations or Warranties |
A-24 |
||||
Annex A-i
|
Page |
||||||
|
Article V REPRESENTATIONS AND WARRANTIES OF ACQUIROR AND MERGER SUB |
A-24 |
|||||
|
Section 5.01 |
Organization, Standing and Corporate Power |
A-24 |
||||
|
Section 5.02 |
Corporate Authority; Approval; Non-Contravention; Government Approvals |
A-25 |
||||
|
Section 5.03 |
Compliance with Laws |
A-25 |
||||
|
Section 5.04 |
Employee Benefit Plans |
A-25 |
||||
|
Section 5.05 |
Indebtedness |
A-26 |
||||
|
Section 5.06 |
Taxes |
A-26 |
||||
|
Section 5.07 |
Brokers |
A-27 |
||||
|
Section 5.08 |
Acquiror SEC Reports; Financial Statements; Sarbanes-Oxley Act |
A-27 |
||||
|
Section 5.09 |
Business Activities; Absence of Changes |
A-28 |
||||
|
Section 5.10 |
Information Supplied; Information Statement |
A-29 |
||||
|
Section 5.11 |
Litigation |
A-29 |
||||
|
Section 5.12 |
No Outside Reliance |
A-29 |
||||
|
Section 5.13 |
Capitalization |
A-29 |
||||
|
Section 5.14 |
NYSE American Quotation |
A-30 |
||||
|
Section 5.15 |
Affiliate Agreements |
A-30 |
||||
|
Section 5.16 |
Anti-Bribery; Economic Sanctions |
A-30 |
||||
|
Section 5.17 |
Labor and Employment |
A-31 |
||||
|
Section 5.18 |
No Other Representations or Warranties |
A-32 |
||||
|
Article VI COVENANTS OF THE COMPANY |
A-32 |
|||||
|
Section 6.01 |
Financial Statements; Stockholder Approval; Other Actions |
A-32 |
||||
|
Section 6.02 |
Asset Covenant |
A-33 |
||||
|
Article VII COVENANTS OF ACQUIROR |
A-33 |
|||||
|
Section 7.01 |
Access and Information |
A-33 |
||||
|
Section 7.02 |
Indemnification and Insurance |
A-33 |
||||
|
Section 7.03 |
Additional Insurance Matters |
A-34 |
||||
|
Section 7.04 |
Conduct of Business |
A-34 |
||||
|
Section 7.05 |
Director and Officer Appointments |
A-36 |
||||
|
Section 7.06 |
Written Consent |
A-36 |
||||
|
Section 7.07 |
Post-Closing Governance of the Surviving Corporations |
A-37 |
||||
|
Section 7.08 |
Bio Sub and Financing Allocations |
A-37 |
||||
|
Section 7.09 |
Reserved |
A-39 |
||||
|
Section 7.10 |
NYSE American Listing Application |
A-39 |
||||
|
Section 7.11 |
Form S-8 Filing Related to 2024 Plan Share Increase |
A-39 |
||||
|
Article VIII joint COVENANTS |
A-39 |
|||||
|
Section 8.01 |
Support of Transaction |
A-39 |
||||
|
Section 8.02 |
Preparation of the Information Statement |
A-39 |
||||
|
Section 8.03 |
Tax Matters |
A-40 |
||||
|
Section 8.04 |
Confidentiality; Publicity |
A-40 |
||||
|
Section 8.05 |
Notification of Certain Matters |
A-41 |
||||
|
Section 8.06 |
Post-Closing Cooperation |
A-41 |
||||
Annex A-ii
|
Page |
||||||
|
Article IX INTENTIONALLY OMITTED. |
A-41 |
|||||
|
Article X MISCELLANEOUS |
A-41 |
|||||
|
Section 10.01 |
Notices |
A-41 |
||||
|
Section 10.02 |
Assignment |
A-42 |
||||
|
Section 10.03 |
Rights of Third Parties |
A-42 |
||||
|
Section 10.04 |
Expenses |
A-42 |
||||
|
Section 10.05 |
Governing Law |
A-42 |
||||
|
Section 10.06 |
Captions; Counterparts |
A-42 |
||||
|
Section 10.07 |
Schedules and Exhibits |
A-43 |
||||
|
Section 10.08 |
Entire Agreement |
A-43 |
||||
|
Section 10.09 |
Amendments |
A-43 |
||||
|
Section 10.10 |
Severability |
A-43 |
||||
|
Section 10.11 |
Jurisdiction; WAIVER OF TRIAL BY JURY |
A-43 |
||||
|
Section 10.12 |
Enforcement |
A-43 |
||||
|
Section 10.13 |
Non-Recourse |
A-44 |
||||
|
Section 10.14 |
Non-survival of Representations, Warranties and Covenants |
A-44 |
||||
|
Section 10.15 |
Acknowledgements |
A-44 |
||||
Annex A-iii
AGREEMENT AND PLAN OF MERGER
This Agreement and Plan of Merger (this “Agreement”), dated as of December 1, 2025, is entered into by and among Apimeds Pharmaceuticals US, Inc., a Delaware corporation (“Acquiror”), Apimeds Merger Sub, Inc., a Delaware corporation (“Merger Sub” and, together with the Acquiror, the “Acquiror Parties”), MindWave Innovations Inc, a Delaware corporation (the “Company”), Lokahi Therapeutics, Inc., a Nevada corporation (“Bio Sub”), and Erik Emerson, solely in his capacity as representative for the Bio Business (the “Bio Business Representative”). Except as otherwise indicated, capitalized terms used but not defined herein shall have the meanings set forth in Article I of this Agreement or as otherwise defined throughout this Agreement.
RECITALS
WHEREAS, the Board of Directors of the Acquiror (the “Acquiror Board”) has determined that it is in the best interest of the Acquiror and its stockholders to consummate the transactions provided for in this Agreement, pursuant to which Merger Sub will, subject to the terms and conditions set forth herein, merge with and into the Company (the “Merger”), so that the Company is the surviving corporation in the Merger (hereinafter sometimes referred to in such capacity as the “Surviving Corporation”);
WHEREAS, the respective board of directors of each of Acquiror, Merger Sub and the Company have each approved and declared advisable this Agreement and the Merger upon the terms and subject to the conditions of this Agreement and in accordance with the Laws of its jurisdiction and the respective board of directors have recommended the approval and adoption of this Agreement and the transactions contemplated thereby, including the Merger, by the equity holders of each of Acquiror, Merger Sub and the Company; and
WHEREAS, contemporaneously with the execution and delivery of this Agreement, in connection with the Merger, certain stockholders of Acquiror (the “Acquiror Stockholders”) constituting a majority of the outstanding voting power of the Acquiror Stockholders (the “Majority Holders”) have entered into a certain Support Agreement, dated as of the date hereof (the “Acquiror Support Agreement”), with the Company and the Acquiror, pursuant to which, among other things, the Majority Holders have agreed to execute a written consent (the “Written Consent”) approving the following matters (collectively, the “Proposals”): (i) the approval of the conversion of the Series A Convertible Preferred Stock, par value $0.01 per share of the Acquiror (the “Acquiror Preferred Stock”) and the issuance of common stock, par value $0.01 per share of the Acquiror (the “Acquiror Common Stock”), upon conversion of the shares of Acquiror Preferred Stock in accordance with the rules of the NYSE American LLC (“NYSE American”) (the “Issuance Proposal”), (ii) the approval of a reverse stock split (the “Reverse Stock Split”) of the Acquiror Common Stock at a ratio of 1-for-10 (the “Reverse Stock Split Proposal”), (iii) the approval of an amendment (the “Charter Amendment”) to the Acquiror’s Amended and Restated Certificate of Incorporation (as amended, the “Acquiror Charter”) (the “Charter Amendment Proposal”), (iv) the approval of an amendment to the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan (the “2024 Plan”) to increase the number of shares of Acquiror Common Stock issuable under the 2024 Plan to 2,096,679 (the “2024 Plan Share Increase”), and (v) the approval and adoption of the Apimeds Pharmaceuticals US, Inc. 2025 Equity Incentive Plan (the “Acquiror Equity Incentive Plan”) (the “Acquiror Equity Plan Proposal”).
NOW, THEREFORE, in consideration of the foregoing and the respective representations, warranties, covenants and agreements set forth in this Agreement, and intending to be legally bound hereby, Acquiror, Merger Sub, the Company, Bio Sub and Bio Business Representative agree as follows:
Article I
CERTAIN DEFINITIONS
Section 1.01 Definitions. As used herein, the following terms shall have the following meanings:
“2024 Plan” has the meaning specified in the Recitals.
“2024 Plan Share Increase” has the meaning specified in the Recitals.
“Acquiror” has the meaning specified in the Preamble hereto.
“Acquiror Equity Incentive Plan” has the meaning specified in the Recitals.
“Acquiror Equity Plan Proposal” has the meaning specified in the Recitals.
Annex A-1
“Acquiror and Merger Sub Representations” means the representations and warranties of each of Acquiror and Merger Sub expressly and specifically set forth in Article V of this Agreement, as qualified by the Acquiror Disclosure Schedules. For the avoidance of doubt, the Acquiror and Merger Sub Representations are solely made by Acquiror and Merger Sub.
“Acquiror Board” has the meaning specified in the Recitals hereto.
“Acquiror Bylaws” means the bylaws, as amended, of the Acquiror.
“Acquiror Charter” has the meaning specified in the Recitals hereto.
“Acquiror Common Stock” has the meaning specified in the Recitals hereto.
“Acquiror Disclosure Schedules” means the disclosure schedules of the Acquiror.
“Acquiror Equity Incentive Plan” has the meaning specified in the Recitals hereto.
“Acquiror Equity Plan Proposal” has the meaning specified in the Recitals hereto.
“Acquiror Material Adverse Effect” means any event, change, circumstance or development (each an “Effect”) that, individually or in the aggregate with all other Effects, that has had or would reasonably be expected to have (a) a material adverse effect on the financial condition, assets, liabilities, business, or results of operations of Acquiror and Merger Sub, taken as a whole, or (b) a prevention, material delay or material impairment in the ability of Acquiror or Merger Sub to timely consummate the Transactions; provided, however, that, solely with respect to clause (a), none of the following (or the effect of any of the following) shall be deemed to constitute, alone or in combination, or be taken into account in the determination of whether, there has been or will be an Acquiror Material Adverse Effect: (i) any change or proposed change in or change in the interpretation of any Law or GAAP; (ii) events or conditions generally affecting the industries or geographic areas in which Acquiror operates; (iii) any downturn in general economic conditions, including changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market index or commodity or any disruption of such markets); (iv) any geopolitical conditions, outbreak of hostilities, acts of war, sabotage, embargo, civil unrest, cyberterrorism, terrorism, military actions, earthquakes, volcanic activity, hurricanes, tsunamis, tornadoes, floods, mudslides, wild fires or other natural disasters, weather conditions, epidemics, pandemics or other outbreaks of illness or public health events and other force majeure events (including any escalation or general worsening of any of the foregoing Effects); (v) any actions taken or not taken by Acquiror as required by this Agreement; (vi) any Effect attributable to the announcement or execution, pendency, negotiation or consummation of the Merger or any of the other Transactions; (vii) any actions taken, or failures to take action, or such other changes or events, in each case, which the Company has requested or to which it has consented or which actions are contemplated by this Agreement; or (viii) any event, circumstance, change or effect arising from or related to the exercise of redemption rights by holders of Acquiror Common Stock, except in the cases of clauses (i) through (iii), to the extent that Acquiror is disproportionately affected thereby as compared with other participants in the industry in which Acquiror operates.
“Acquiror Organizational Documents” means the Acquiror Charter and Acquiror Bylaws.
“Acquiror Parties” has the meaning specified in the Preamble hereto.
“Acquiror Preferred Stock” has the meaning specified in the Recitals hereto.
“Acquiror SEC Reports” has the meaning specified in Section 5.08(a).
“Acquiror Stockholder” means a holder of any Acquiror Common Stock.
“Acquiror Stockholder Approvals” means with respect to each Proposal, the approval evidenced by the affirmative vote of Acquiror Stockholders holding a majority of the issued shares of Acquiror Common Stock, as set forth in the Written Consent.
“Acquiror Support Agreement” has the meaning specified in the Recitals hereto.
“Action” means any claim, action, suit, charge, complaint, assessment, audit, investigation, examination, arbitration, inquiry, dispute, litigation, or proceeding, in each case that is by or before any Governmental Authority.
“Action Effective Time” has the meaning specified in Section 7.06.
Annex A-2
“Affiliate” means, with respect to any specified Person, any Person that, directly or indirectly, controls, is controlled by, or is under common control with, such specified Person, the ownership of voting securities, its capacity as a manager, sole or managing member or otherwise, through one or more intermediaries, where “control” means possession, directly or indirectly, of the power to direct the management and policies of such specified Person.
“Agreement” has the meaning specified in the Preamble hereto.
“Antitrust Law” means (a) the HSR Act, the Federal Trade Commission Act, the Sherman Antitrust Act of 1890, the Clayton Antitrust Act, in each case, including the rules and regulations promulgated thereunder, (b) any applicable foreign antitrust Laws and (c) all other applicable Laws that are designed or intended to prohibit, restrict or regulate actions having the purpose or effect of monopolization or restraint of trade or lessening of competition through merger or acquisition.
“Asset Representation” has the meaning specified in Section 4.26.
“Balance Sheet Date” means March 31, 2025.
“Benefit Plan” means any benefit or compensation plan, program, policy, practice, agreement, Contract, arrangement or other obligation, whether or not in writing and whether or not funded, including, but not limited to, “employee benefit plans” within the meaning of Section 3(3) of ERISA (whether or not subject to ERISA), “voluntary employees’ beneficiary associations,” under Section 501(c)(9) of the Code, employment, individual consulting, retirement, severance, termination pay, change in control, transaction or retention arrangements, deferred compensation, equity or equity-based compensation, incentive compensation, bonus, supplemental retirement, profit sharing, health, medical, welfare, vacation, paid time off, post-termination or retiree health or welfare, fringe or other benefits or remuneration plan.
“Bio Business” has the meaning specified in Section 7.08(a).
“Bio Business Representative” has the meaning specified in the Preamble hereto.
“Bio Sub” has the meaning specified in the Preamble hereto.
“Bio Sub Loan” has the meaning specified in Section 7.08(g).
“Business Day” means a day other than a Saturday, Sunday or other day on which commercial banks in New York, New York are authorized or required by Law to close.
“Calfin” has the meaning specified in Section 4.26.
“CBA” has the meaning set forth in Section 4.11(a)(xiv).
“Certificate of Designation” means the Certificate of Designation of the Acquiror for the Acquiror Preferred Stock, as filed with the Secretary of State of the State of Delaware on December 1, 2025.
“Certificate of Merger” has the meaning specified in Section 2.01.
“Charter Amendment” has the meaning specified in the Recitals hereto.
“Charter Amendment Proposal” has the meaning specified in the Recitals hereto.
“Closing” has the meaning specified in Section 2.03.
“Closing Date” has the meaning specified in Section 2.03.
“Code” means the Internal Revenue Code of 1986.
“Common Stock Cap” has the meaning specified in Section 3.01(b).
“Company” has the meaning specified in the Preamble hereto.
“Company Benefit Plan” means any Benefit Plan which is sponsored or maintained by, contributed to or required to be contributed to by, or with respect to or under which any current or potential liability or obligation is borne by the Company.
Annex A-3
“Company Board” means the board of directors of the Company.
“Company Common Stock” means the “Common Stock” of the Surviving Corporation.
“Company Disclosure Schedules” means the disclosure schedules of the Company.
“Company Intellectual Property” means all Owned Intellectual Property and all other Intellectual Property used in the business of the Company, as currently conducted.
“Company Material Adverse Effect” means any Effect that, individually or in the aggregate with one or more other Effects, (a) is or would be reasonably expected to be materially adverse to the business, financial condition or results of operations of the Company or (b) the ability of the Company to consummate the Transactions; provided, however that none of the following shall be deemed to constitute, alone or in combination, or be taken into account in the determination of whether, there has been or will be a Company Material Adverse Effect: (i) any change in or change in the interpretation of any applicable Laws or GAAP, (ii) any events or conditions generally affecting any industry or geographic area in which the Company operates, (iii) any downturn in general economic conditions, including changes in the credit, debt, securities, financial or capital markets (including changes in interest or exchange rates, prices of any security or market index or commodity or any disruption of such markets), (iv) any geopolitical conditions, outbreak of hostilities, acts of war, sabotage, embargo, civil unrest, cyberterrorism, terrorism, military actions, earthquakes, volcanic activity, hurricanes, tsunamis, tornadoes, floods, mudslides, wild fires or other natural disasters, weather conditions, epidemics, pandemics or other outbreaks of illness or public health events and other force majeure events (including any escalation or general worsening of any of the foregoing Effects), (v) any actions taken or not taken by any Company as required by this Agreement or the Acquiror Support Agreement, (vi) any Effect attributable to the announcement or execution, pendency or consummation of the Merger or the performance of this Agreement (including the impact thereof on relationships with customers, suppliers, licensors, distributors, partners, providers and employees) (provided, that this clause (vi) shall not apply to any representation or warranty in Sections 4.2, 4.3, 4.6, 4.11 and 4.23 of this Agreement, but subject to the disclosures set forth on Schedules 4.2, 4.3, 4.6, 4.11 and 4.23), (vii) any failure to meet any projections, forecasts or budgets; provided that this clause (vii) shall not prevent a determination that any Effect underlying such failure has resulted in a Company Material Adverse Effect, or (viii) any actions taken, or failures to take action, or such other changes or events, in each case, which Acquiror has consented to in writing prior to the taking of, or failure to take, such action, except in the cases of clauses (i) through (iv) to the extent the Company is as a whole materially disproportionately affected thereby as compared with other participants in the industry in which the Company operates.
“Company Organizational Documents” means the Existing Company Charter and the Existing Company Bylaws.
“Company Permits” has the meaning specified in Section 4.06(c).
“Company Representations” means the representations and warranties of the Company expressly and specifically set forth in Article IV of this Agreement, as qualified by the Company Disclosure Schedules. For the avoidance of doubt, the Company Representations are solely made by the Company.
“Company Stockholder” means, as of any particular time, the holder of Existing Company Stock.
“Company Stockholder Approval” has the meaning specified Section 6.01(c).
“Company Software” means all Software with respect to which all Intellectual Property embodied thereby are owned or purported to be owned by the Company.
“Confidential Information” means any proprietary or confidential information concerning the Company, Acquiror or the business and affairs of either party or information not already generally available to the public.
“Contracts” means any legally binding contracts, agreements, subcontracts, licenses, leases, and purchase orders.
“Conversion” has the meaning specified in Section 3.05.
“Conversion Effective Time” means the time the Conversion is effective.
“Definitive Information Statement” has the meaning specified in Section 7.06.
Annex A-4
“DGCL” means the General Corporation Law of the State of Delaware, as amended from time to time.
“Director Proposal” has the meaning set forth in Section 8.02(c).
“Effect” has the meaning specified in the definition of “Acquiror Material Adverse Effect.”
“Effective Time” has the meaning specified in Section 2.01.
“Enforceability Exceptions” has the meaning specified in Section 4.02(a).
“Environmental Laws” means all Laws relating to pollution or protection of the environment (including natural resources), health and safety (to the extent relating to management of or exposure to Hazardous Materials), or the use, generation, storage, emission, transportation, disposal or release of or exposure to Hazardous Materials.
“Equity Securities” means any share, share capital, capital stock, partnership, membership, joint venture or similar interest in any Person (including any stock appreciation right, phantom stock, restricted stock unit, performance stock unit, restricted stock, profit participation or similar rights) and any option, warrant, right or security (including debt securities) convertible, exchangeable or exercisable therefor.
“ERISA” means the Employee Retirement Income Security Act of 1974, as amended.
“ERISA Affiliate” means, with respect to the Company, any other entity, trade or business that is a member of a group described in Section 414(b),(c), (m) or (o) of the Code or Section 4001(b)(l) of ERISA that includes the Company, or that is a member of the same “controlled group” as the first entity, trade or business pursuant to Section 4001(a)(14) of ERISA.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Existing Company Bylaws” means the bylaws of the Company prior to the Effective Time.
“Existing Company Charter” means the certificate of incorporation of the Company prior to the Effective Time.
“Existing Company Common Stock” means the “Common Stock” of the Company (as defined in the Existing Company Charter).
“Existing Company Preferred Stock” means the “Preferred Stock” of the Company (as defined in the Existing Company Charter).
“Existing Company Stock” means the Existing Company Common Stock and the Existing Company Preferred Stock.
“Financial Derivative/Hedging Arrangement” means any transaction (including an agreement with respect thereto) which is a rate swap transaction, basis swap, forward rate transaction, commodity swap, commodity option, equity or equity index swap, equity or equity index option, bond option, interest rate option, foreign exchange transaction, cap transaction, floor transaction, collar transaction, currency swap transaction, cross-currency rate swap transaction, currency option or any combination of these transactions.
“Financial Statements” has the meaning specified in Section 4.05(a).
“Form 8-K” has the meaning set forth in Section 8.04(b).
“Funding Cap” means $14,800,000.
“GAAP” means generally accepted accounting principles in the United States set forth in the opinions and pronouncements of the Financial Accounting Standards Board or such other principles as may be approved by a significant segment of the accounting profession in the United States, that are applicable to the circumstances as of the date of determination, consistently applied.
“Governmental Authority” means any U.S. or foreign federal, state, provincial, municipal, local or foreign government, governmental authority, regulatory or administrative agency, governmental commission, department, board, bureau, agency or instrumentality, arbitrator or arbitral body (public or private), court, tribunal any state-owned or controlled enterprise.
Annex A-5
“Governmental Order” means any order, judgment, injunction, decree, writ, stipulation, determination or award, in each case, entered by or with any Governmental Authority.
“Hazardous Material” means any material, substance or waste that is listed, regulated, or defined as “hazardous,” “toxic,” or “radioactive,” or as a “pollutant” or “contaminant” (or words of similar intent or meaning) under applicable Environmental Laws, including but not limited to petroleum, petroleum by-products, asbestos or asbestos-containing material, polychlorinated biphenyls, per- and pol-fluoroalkyl substances, flammable or explosive substances, toxic mold or pesticides.
“Indebtedness” means, with respect to any Person, without duplication, any obligations (whether or not contingent) consisting of (a) the outstanding principal amount of and accrued and unpaid interest on, and other payment obligations for, borrowed money, or payment obligations issued or incurred in substitution or exchange for payment obligations for borrowed money, (b) amounts owing as deferred purchase price for property or services, including “earnout” payments (but excluding ordinary trade accounts payable), (c) payment obligations evidenced by any promissory note, bond, debenture, mortgage or other debt instrument or debt security, (d) contingent reimbursement obligations with respect to letters of credit, bankers’ acceptance or similar facilities (in each case to the extent drawn), (e) any obligations in the nature of accrued fees, interest, prepayment or other premiums, penalties, termination fees, expenses and other amounts incurred or that would be payable in connection with the prepayment, repayment, redemption, payoff, amendment, modification or supplement of any of the items in the foregoing clauses, (f) payment obligations of a third party secured by (or for which the holder of such payment obligations has an existing right, contingent or otherwise, to be secured by) any Lien, other than a Permitted Lien, on assets or properties of such Person, whether or not the obligations secured thereby have been assumed, (g) obligations under capitalized leases, (h) obligations net of benefits under all Financial Derivative/Hedging Arrangements, (i) any underfunded pension liability, unfunded deferred compensation plan obligations, and post-retirement health or welfare benefits, (j) any unpaid dividends or distributions declared or payable to any Company Stockholder, (k) any other indebtedness or obligation reflected or required to be reflected as indebtedness in a consolidated balance sheet, in accordance with GAAP, (l) guarantees, make-whole agreements, hold harmless agreements or other similar arrangements with respect to any amounts of a type described in the foregoing clauses, and (m) with respect to each of the foregoing, any unpaid interest, breakage costs, prepayment or redemption penalties or premiums, or other unpaid fees or obligations (including unreimbursed expenses or indemnification obligations for which a claim has been made); provided, however, that Indebtedness shall not include Taxes or accounts payable to trade creditors in the ordinary course of business that are not past due and accrued expenses arising in the ordinary course of business consistent with past practice.
“Issuance Proposal” has the meaning specified in the Recitals.
“Proposals” has the meaning specified in the Recitals hereto.
“Information Statement” has the meaning specified in Section 8.02(a).
“Insurance Policies” has the meaning specified in Section 4.20(a).
“Intellectual Property” means all intellectual property rights, as they exist anywhere in the world, whether registered or unregistered, including all: (a) patents and patent applications (including any divisions, continuations, continuations-in-part, reissues, reexaminations and interferences thereof); (b) trademarks, service marks, trade dress, trade names, brand names, logos and corporate names; (c) copyrights, mask works and designs; (d) internet domain names; (e) trade secrets and other intellectual property rights in know-how, technology, inventions (whether patentable or not), processes, procedures, database rights, confidential business information and other proprietary information and rights; and (f) intellectual property rights in Software.
“Intended Tax Treatment” has the meaning specified in Section 8.03(b).
“Interim Period” has the meaning specific in Section 7.01.
“IT Systems” means all computer hardware (including hardware, firmware, peripherals, communication equipment and links, storage media, networking equipment, power supplies and any other components used in conjunction with such), data processing systems, Software, and all other information technology equipment owned or controlled by the Company and/or used in the operation of the Company business.
Annex A-6
“Knowledge” shall mean the actual knowledge of (a) in the case of the Company, Dr. Vin Menon and Yadav Sandeep Singh and (b) in the case of Acquiror, Erik Emerson and Erick Frim.
“Law” means any statute, law (including common law), act, constitution, treaty, code, ordinance, rule, ruling, regulation or Governmental Order, in each case, of any Governmental Authority. All references to “Laws” shall be deemed to include any amendments thereto, and any successor Law, unless the context otherwise requires.
“Lease Documents” has the meaning specified in Section 4.18(c).
“Leased Company Properties” has the meaning specified in Section 4.18(b).
“Lien” means any mortgage, deed of trust, pledge, hypothecation, easement, right of way, purchase option, right of first refusal, covenant, restriction, security interest, license, title defect, encroachment or other survey defect, or other lien or encumbrance of any kind, except for (a) any restrictions arising under any applicable Securities Laws, and (b) immaterial easements, rights of way, covenants, encumbrances or restrictions that do not materially detract the value of the underlying asset or the use of the asset.
“Majority Holders” has the meaning specified in the Recitals.
“Material Contracts” has the meaning specified in Section 4.11(a).
“Material Customers” has the meaning specified in Section 4.23(a).
“Material Vendors” has the meaning specified in Section 4.23(b).
“Merger” has the meaning specified in the Recitals hereto.
“Merger Consideration” has the meaning specified in Section 3.01(b).
“Merger Sub” has the meaning specified in the Preamble hereto.
“NYSE American” has the meaning specified in the Recitals hereto.
“Open Source Software” means software that is distributed as “free software” (as defined by the Free Software Foundation), “open source software” (meaning software distributed under any license approved by the Open Source Initiative as set forth at www.opensource.org) or under a similar licensing or distribution model (including under a GNU General Public License (GPL), a GNU Lesser General Public License (LGPL), a Mozilla Public License (MPL), a BSD license, an Artistic License, a Netscape Public License, a Sun Community Source License (SCSL), a Sun Industry Standards License (SISL), and/or an Apache License).
“Owned Intellectual Property” means all Intellectual Property owned or purported to be owned by the Company.
“Permitted Liens” means (a) statutory or common law Liens of mechanics, materialmen, warehousemen, landlords, carriers, repairmen, construction contractors and other similar Liens that arise in the ordinary course of business, and (i) relate to amounts not yet delinquent or (ii) that are being contested in good faith through appropriate Actions and for which appropriate reserves for the amount being contested have been established in accordance with GAAP on the Financial Statements, (b) Liens arising under original purchase price conditional sales contracts and equipment leases with third parties entered into in the ordinary course of business, (c) Liens for Taxes not yet due and payable or which are being contested in good faith through appropriate Actions, and for which appropriate reserves have been established in accordance with GAAP on the Financial Statements, (d) non-monetary Liens, encumbrances and restrictions on real property (including easements, covenants, rights of way and similar restrictions) of record affecting title to real property that do not, individually or in the aggregate, materially interfere with the occupancy or present uses of such real property, (e) non-exclusive licenses of Intellectual Property, (f) requirements and restrictions of zoning, building and other applicable Laws and municipal by-laws, and development, site plan, subdivision or other agreements with municipalities, which do not materially interfere with the current use or occupancy of any Leased Company Properties, and (g) Liens that do not, individually or in the aggregate, materially and adversely affect, or materially disrupt, the ordinary course operation of the businesses of the Company, taken as a whole.
“Person” means any individual, firm, corporation, partnership, limited liability company, incorporated or unincorporated association, joint venture, joint stock company, Governmental Authority or other entity of any kind.
Annex A-7
“Personal Information” means any personal information that specifically identifies any individual who has provided information to the Company, including names, addresses, telephone numbers, personal health information, drivers’ license numbers and government-issued identification numbers, as applicable.
“Premium Cap” has the meaning specified in Section 7.02.
“Press Release” has the meaning set forth in Section 8.04(b).
“Privacy Laws” means any and all Laws applicable to the Company relating to the collection, use, storage, safeguarding and security (both technical and physical) of Personal Information.
“Private Placement Offering” has the meaning specified in Section 7.08(d).
“Process” means the access, creation, collection, use, storage, maintenance, processing, recording, sharing, distribution, transfer, transmission, receipt, import, export, protection, safeguarding, access, disposal or disclosure or other activity regarding data (whether electronically or in any other form or medium).
“Products” mean any products or services, developed, manufactured, performed, out-licensed, sold, distributed or otherwise made available by or on behalf of the Company, from which the Company has derived previously, is currently deriving, or is scheduled to derive revenue from the sale or provision thereof.
“Public Official” means (a) any director, manager, officer, employee or representative of any Governmental Authority; (b) any director, manager, officer, employee or representative of any commercial enterprise that is owned or controlled by a Governmental Authority; (c) any director, manager, officer, employee or representative of any public international organization; (d) any Person acting in an official capacity for or on behalf of any Governmental Authority; and (e) any political party, party official or candidate for political office.
“Registered IP” has the meaning specified in Section 4.15(a).
“Representative” means, as to any Person, any of the officers, directors, managers, employees, counsel, accountants, disclosed financial advisors, disclosed lenders, disclosed debt financing sources and consultants of such Person.
“Reverse Stock Split” has the meaning specified in the Recitals.
“Reverse Stock Split Proposal” has the meaning specified in the Recitals.
“Sanctioned Country” has the meaning specified in the definition of “Sanctioned Person.”
“SEC” means the United States Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended.
“Securities Laws” means the securities laws of any state, U.S. federal or foreign jurisdiction and the rules and regulations promulgated thereunder.
“Service Agreements” has the meaning specified in Section 4.12(d).
“Side Letter” has the meaning specified in Section 7.08(e).
“Software” means any and all (a) computer programs, including any and all software implementation of algorithms, models and methodologies, whether in source code, object code, human readable form or other form, (b) databases and compilations, including any and all data and collections of data, whether machine readable or otherwise, (c) descriptions, flow charts and other work products used to design, plan, organize and develop any of the foregoing, screens, user interfaces, report formats, firmware, development tools, templates, menus, buttons and icons and (d) all documentation including user manuals and other training documentation relating to any of the foregoing.
“Subsidiary” means, with respect to a Person, any corporation or other organization (including a limited liability company or a partnership), whether incorporated or unincorporated, of which such Person directly or indirectly owns or controls a majority of the Equity Securities.
“Surviving Corporation” has the meaning specified in the Recitals.
Annex A-8
“Tax” means any federal, state, provincial, territorial, local, foreign and other net income, alternative or add-on minimum, franchise, gross income, adjusted gross income or gross receipts, employment, environmental, unemployment, compensation, utility, social security (or similar), withholding, payroll, ad valorem, transfer, windfall profits, license, branch, excise, severance, production, stamp, occupation, premium, personal property, real property, capital stock, profits, disability, registration, value added, capital gains, goods and services, estimated, sales, use, unclaimed property or escheat obligation, or other tax, governmental fee, duty, charge, impost, or assessment of any kind whatever in the nature of a tax, whether disputed or not, together with any interest, deficiency, penalty, addition to tax or additional amount imposed with respect thereto by a Governmental Authority.
“Tax Authority” means any Governmental Authority with jurisdiction or authority to impose, administer, levy, assess or collect Tax.
“Tax Return” means any return, report, statement, refund, claim, election, disclosure, declaration, information report or return, estimate or other document filed or required to be filed with a Tax Authority with respect to Taxes, including any schedule or attachment thereto and including any amendments thereof.
“Transaction Expenses” means any fees, costs and expenses incurred or subject to reimbursement by the Company, Acquiror or Merger Sub, whether accrued for or not, in each case in connection with the Transactions contemplated by this Agreement and the Acquiror Support Agreement, including (a) any brokerage fees, commissions, finders’ fees, or financial advisory fees, and, in each case, related costs and expenses, (b) any fees, costs and expenses of counsel, accountants or other advisors or service providers, and (c) with respect to Acquiror and Merger Sub, any fees, costs and expenses or payments related to any transaction bonus, discretionary bonus, change-of-control payment, retention or other compensatory payments made to any employee of the Acquiror or Merger Sub solely as a result or related to (and measured assuming the satisfaction of any other related contingencies such as termination or the passage of time) of the execution of this Agreement or the Acquiror Support Agreement or the consummation of the transactions contemplated hereby and thereby (including the employer portion of any payroll, social security, unemployment or similar Taxes imposed with respect thereto). For the avoidance of doubt, no bonus, change-of-control payment, retention or other compensatory payment paid to any manager, officer or employee of the Company shall be a Transaction Expense.
“Transactions” means the transactions contemplated by this Agreement, including the Merger.
“Transfer Taxes” has the meaning specified in Section 8.03(a).
“Treasury Regulations” means the U.S. Treasury Department regulations promulgated under the Code.
“Waiting Period” has the meaning specified in Section 7.06.
“Written Consent” has the meaning specified in the Recitals hereto.
Section 1.02 Construction.
(a) Unless the context of this Agreement otherwise requires, (i) words of any gender include each other gender, (ii) words using the singular or plural number also include the plural or singular number, respectively, (iii) the terms “hereof,” “herein,” “hereby,” “hereto” and derivative or similar words refer to this entire Agreement, (iv) the terms “Article,” “Section,” “Schedule,” “Exhibit” and “Annex” refer to the specified Article, Section, Schedule, Exhibit or Annex of or to this Agreement unless otherwise specified, (v) the word “including” shall mean “including without limitation,” (vi) the word “or” shall be disjunctive but not exclusive and (vii) any reference to a Law shall mean such Law as amended.
(b) Unless the context of this Agreement otherwise requires, references to agreements and other documents shall be deemed to include all subsequent amendments, waivers and other modifications thereto.
(c) Unless the context of this Agreement otherwise requires, references to statutes shall include all regulations promulgated thereunder and references to statutes or regulations shall be construed as including all statutory and regulatory provisions consolidating, amending or replacing the statute or regulation.
(d) The language used in this Agreement shall be deemed to be the language chosen by the parties to express their mutual intent and no rule of strict construction shall be applied against any party.
Annex A-9
(e) Whenever this Agreement refers to a number of days, such number shall refer to calendar days unless Business Days are specified. If any action is to be taken or given on or by a particular calendar day, and such calendar day is not a Business Day, then such action may be deferred until the next Business Day.
(f) All accounting terms used herein and not expressly defined herein shall have the meanings given to them under GAAP.
(g) The phrases “delivered,” “provided to,” “furnished to,” “made available” and phrases of similar import when used herein, unless the context otherwise requires, means that a copy of the information or material referred to has been provided no later than two (2) Business Days prior to the date of this Agreement to the party to which such information or material is to be provided or furnished (i) in the virtual “data room” set up by the Company in connection with this Agreement or (ii) by delivery to such party or its legal counsel via electronic mail or hard copy form.
Article II
THE MERGER; CLOSING
Section 2.01 The Merger. Upon the terms and subject to the conditions set forth in this Agreement, at the Effective Time, Merger Sub shall be merged with and into the Company, with the Company being the Surviving Corporation following the Merger and shall continue its corporate existence under the laws of Delaware as a wholly owned subsidiary of Acquiror, and the separate existence of Merger Sub shall cease. The Merger shall be consummated in accordance with this Agreement and the DGCL and evidenced by a certificate of merger (the “Certificate of Merger”), such Merger to be consummated and effective upon the filing of the Certificate of Merger with the Secretary of State of the State of Delaware or at such later time as may be agreed by Acquiror and the Company in writing and specified in the Certificate of Merger (the “Effective Time”).
Section 2.02 Effects of the Merger. The Merger shall have the effects set forth in this Agreement and the DGCL. Without limiting the generality of the foregoing and subject thereto, by virtue of the Merger and without further act or deed, at the Effective Time, all of the property, rights, privileges, powers and franchises of the Company and Merger Sub shall vest in the Surviving Corporation and all of the debts, liabilities and duties of the Company and Merger Sub shall become the debts, liabilities and duties of the Surviving Corporation.
Section 2.03 Closing. Subject to the terms and conditions of this Agreement, the closing of the Merger (the “Closing”) shall take place electronically through the exchange of documents via e-mail or facsimile simultaneously with the execution and delivery of this Agreement. Subject to the satisfaction or waiver of all of the conditions set forth in Article IX of this Agreement, and provided this Agreement has not theretofore been terminated pursuant to its terms, on the Closing Date, the Company, Acquiror and Merger Sub shall cause the Certificate of Merger to be executed, acknowledged and filed with the Secretary of State of Delaware as provided in the DGCL. The date on which the Closing actually occurs is referred to in this Agreement as the “Closing Date.”
Section 2.04 Organizational Documents of the Company and Acquiror.
(a) At the Effective Time, the Existing Company Charter, as in effect immediately prior to the Effective Time, shall be the certificate of incorporation of the Surviving Corporation, until thereafter supplemented or amended as provided therein and in accordance with the DGCL (subject to Section 7.02).
(b) At the Effective Time, the Existing Company Bylaws, as in effect immediately prior to the Effective Time, shall continue to be the bylaws of the Surviving Corporation, until thereafter supplemented or amended in accordance with its terms and the DGCL.
(c) The Acquiror Charter, as in effect immediately prior to the Effective Time, shall be the certificate of incorporation of the Acquiror, until thereafter supplemented or amended in accordance with its terms and the DGCL.
(d) The Acquiror Bylaws, as in effect immediately prior to the Effective Time, shall be the bylaws of the Acquiror, until thereafter supplemented or amended in accordance with its terms and the DGCL.
Annex A-10
Section 2.05 Directors and Officers of the Companies.
(a) Persons constituting the officers of the Company prior to the Effective Time shall continue to be the officers of the Surviving Corporation, in their respective capacities, until the earlier of their death, resignation or removal or until their respective successors are duly appointed.
(b) Intentionally omitted.
(c) Acquiror shall take all necessary actions prior to the Effective Time such that (i) the individual serving as the chief executive officer of the Acquiror immediately prior to the Effective Time shall cease to hold such office as of the Effective Time, (ii) an individual designated by the Company shall be appointed as the chief executive officer of the Acquiror, effective as of the Effective Time, (iii) a new officer position of President shall be created, and Erik Emerson shall be appointed to serve as President of the Acquiror, effective as of the Effective Time, and (iv) all other officers of the Acquiror prior to the Effective Time shall, as of the Effective Time, continue as the officers of Acquiror in identical positions until the earlier of their death, resignation or removal or until their respective successors are duly appointed.
Article III
Effect on SECURITIES
Section 3.01 Effect on Securities. At the Effective Time, by virtue of the Merger and without any action on the part of the Company, Acquiror, Merger Sub or the holder of any Existing Company Stock:
(a) Conversion of Merger Sub Common Stock. Each share of common stock of Merger Sub, issued and outstanding immediately prior to the Effective Time shall be converted into one (1) validly issued, fully paid and nonassessable share of Company Common Stock.
(b) Consideration for All Other Company Capital Stock. At the Effective Time, (i) each share of Existing Company Stock issued and outstanding immediately prior to the Effective Time shall be canceled and converted into the right to receive a portion of the Merger Consideration, consisting of shares of Acquiror Preferred Stock, as set forth in this Section 3.01(b); and (ii) each holder of such shares shall receive, for each share of Existing Company Common Stock held immediately prior to the Effective Time, a pro rata portion of the Merger Consideration, allocated as follows: (A) a number of duly authorized, validly issued, fully paid and nonassessable shares of Acquiror Common Stock, such that the aggregate number of shares of Acquiror Common Stock issued to all holders of Existing Company Common Stock shall equal 0% of the total number of shares of Acquiror Common Stock issued and outstanding as of the date of this Agreement (the “Common Stock Cap”), with each holder’s allocation rounded down to the nearest whole share; and (B) a number of duly authorized, validly issued, fully paid and nonassessable shares of Acquiror Preferred Stock, such that, immediately following the Effective Time, the holders of Existing Company Common Stock collectively hold, on an as-converted to Acquiror Common Stock basis, 90.9% of the total issued and outstanding equity securities of the Acquiror (exclusive of the Acquiror Common Stock issued pursuant to clause (A) and calculated on a fully diluted basis). The shares of Acquiror Common Stock and Acquiror Preferred Stock issued to holders of Existing Company Common Stock pursuant to this Section 3.01(b), on an as-converted and fully diluted basis, shall collectively represent 90.9% of the equity capital of the Acquiror as of the Closing. For purposes of this Agreement, the shares of Acquiror Common Stock, Acquiror Preferred Stock, and Company Common Stock issued pursuant to this Section 3.01 are collectively referred to as the “Merger Consideration.”
Section 3.02 Withholding. Each of Acquiror, Merger Sub, the Company, the Surviving Corporation and their respective Affiliates and agents shall be entitled to deduct and withhold from any amounts otherwise deliverable or payable under this Agreement such amounts that any such Persons are required to deduct and withhold with respect to any of the deliveries and payments contemplated by this Agreement under the Code or any other applicable Law. To the extent that Acquiror, Merger Sub, the Company, the Surviving Corporation or their respective Affiliates withholds or deducts such amounts with respect to any Person and properly remits such withheld or deducted amounts to the applicable Governmental Authority, such withheld or deducted amounts shall be treated as having been paid to or on behalf of such Person in respect of which such withholding or deduction was made for all purposes. In the case of any such payment payable to employees of the Company or its Affiliates in connection with the Merger that is properly treated as compensation, the parties shall cooperate to pay such amounts through the Company’s or an Affiliate’s payroll to facilitate applicable withholding.
Annex A-11
Section 3.03 No Fractional Shares. Notwithstanding anything to the contrary contained herein, no certificates or scrip representing fractional shares of Acquiror Common Stock shall be issued upon the exchange for Existing Company Common Stock pursuant to Section 3.02, and such fractional share interests shall not entitle the owner thereof to vote or to any other rights of a holder of Acquiror Common Stock.
Section 3.04 Reverse Stock Split. As soon as reasonably practicable after the Action Effective Time, the Acquiror shall cause the Charter Amendment to be filed with the Secretary of State of the State of Delaware to implement the Reverse Stock Split, and the Reverse Stock Split shall be effective.
Section 3.05 Conversion of Preferred Stock. As soon as reasonably practicable after the Reverse Stock Split is effective, the Acquiror shall cause the Acquiror Preferred Stock to be converted into the applicable number of shares of Acquiror Common Stock, in accordance with the terms of the Certificate of Designation (the “Conversion”).
Article IV
REPRESENTATIONS AND WARRANTIES OF THE COMPANY
For purposes of this Article IV, all references to the “Company” shall be deemed to include each of the Company and TechyTrade Innovations Pte. Ltd., a company organized and existing under the laws of Singapore and a wholly owned subsidiary of the Company (“TechyTrade”), unless the context otherwise requires. Except as set forth in the Company Disclosure Schedules to this Agreement (each of which qualifies (a) the correspondingly numbered representation, warranty or covenant if specified therein and (b) such other representations, warranties or covenants where its relevance as an exception to (or disclosure for purposes of) such other representation, warranty or covenant is reasonably apparent on its face), the Company represents and warrants to Acquiror and Merger Sub as follows:
Section 4.01 Organization, Standing and Corporate Power.
(a) The Company is a corporation, duly incorporated, and validly existing under the laws of the State of Delaware and has all requisite legal entity power and authority to carry on its business as now being conducted. The Company is duly qualified or licensed to do business and is in good standing in each jurisdiction in which the conduct of its business or the ownership, leasing or operation of its properties makes such qualification or licensing necessary, except as would not, individually or in the aggregate, reasonably be expected to prevent, materially delay or materially impair the ability of the Company to consummate the Transactions or have a Company Material Adverse Effect. The Company Organizational Documents, as amended to the date of this Agreement and that have been made available to the Acquiror are true, correct and complete and are in effect as of the date of the Agreement and the Company is not in material default under or in material violation of any provision thereunder.
Section 4.02 Corporate Authority; Approval; Non-Contravention.
(a) Including the Company Stockholder Approval, the Company has all requisite corporate or other legal entity power and authority and has taken all corporate or other legal entity action necessary in order to execute, deliver and perform its obligations under this Agreement and the Acquiror Support Agreement to which it is a party and, subject to satisfaction of the conditions to Closing contemplated hereby, to consummate the Transactions. The execution, delivery and performance by the Company of this Agreement, the Acquiror Support Agreement, and the consummation by it of the Transactions, have been duly and validly authorized by all necessary corporate consent and authorizations on the part of the Company, and no other corporate actions on the part of the Company are necessary to authorize the execution and delivery by the Company of this Agreement and the consummation by it of the Transactions. This Agreement has been duly executed and delivered by the Company and, assuming due authorization, execution and delivery hereof by the other parties hereto, is a legal, valid and binding obligation of the Company, enforceable against the Company in accordance with its terms (subject to applicable bankruptcy, insolvency, fraudulent transfer, reorganization, moratorium and other Laws affecting creditors’ rights generally from time to time in effect and by general equitable principles (the “Enforceability Exceptions”)).
(b) The execution, delivery and performance of this Agreement and the consummation of the Transactions, do not, and will not, constitute or result in (i) a breach or violation of, or a default under, the Company Organizational Documents or, (ii) with or without notice, lapse of time or both, a breach or violation of, a termination (or right of termination) of or default or change of control under, the creation or acceleration of any obligations under or the creation of a Lien (other than a Permitted Lien) on any of the material assets of the Company pursuant to, any Material Contract to which any member of the Company is a party or, assuming (solely with respect to performance
Annex A-12
of this Agreement and consummation of the Transactions) compliance with the matters referred to in Section 4.02(a), under any Law to which the Company is subject (except Laws that are applicable due to the Company’s business, or the Contracts or licenses of the Company), except as disclosed on Schedule 4.02(b).
Section 4.03 Governmental Approvals. No consent of, or registration, declaration, notice or filing with, any Governmental Authority is required by or with respect to the Company in connection with the execution and delivery by the Company of this Agreement or the consummation of the Transactions, except for such consents, registrations, declarations, notices and filings which, if not obtained or made, would not, individually or in the aggregate, reasonably be expected to be material to the Company.
Section 4.04 Capitalization.
(a) Set forth on Schedule 4.04(a) is a true, correct and complete list of each holder of issued and outstanding Equity Securities (including notes and other securities convertible into Equity Securities) of the Company and the Equity Securities held by each such holder as of the date hereof. Each of the outstanding Equity Securities of the Company (1) is duly authorized, validly issued, fully paid and nonassessable, (2) was issued in compliance in all material respects with applicable Laws, and (3) was not issued in breach or violation of any preemptive rights or Contract. The Company’s Subsidiaries are set forth on Schedule 4.04(a).
(b) Except as set forth in Schedule 4.04(b), there are no preemptive or other outstanding rights, options, warrants, phantom interests, conversion rights, equity appreciation rights, profit participation rights, redemption rights, repurchase rights, agreements, arrangements, calls or commitments of any kind that obligate the Company to issue or to sell any Equity Securities of the Company, or any securities or obligations convertible or exchangeable into or exercisable for, valued by reference to or giving any Person a right to subscribe for or acquire, any Equity Securities of the Company or to vote with the Company Stockholders on any matter, and no securities or obligations evidencing such rights are authorized, issued or outstanding. Except as set forth in Schedule 4.04(b), the Company is not party to any stockholders agreement, voting agreement or registration rights agreement relating to its Equity Securities.
(c) The Company Common Stock to be issued by the Company in connection with the Transactions, upon issuance in accordance with the terms of this Agreement, will be duly authorized, validly issued, fully paid and nonassessable (except as otherwise limited by the Companies Act), and will not be subject to any preemptive rights, free and clear of all Liens (other than restrictions on transfer under applicable Securities Laws and the Existing Company Charter).
Section 4.05 Financial Statements; Internal Controls.
(a) The audited statements of financial position, statements of comprehensive income, statements of changes in stockholders’ equity and statements of cash flows of the Company for the year ended March 31, 2025 (collectively, the “Financial Statements”), were prepared and audited in accordance with the standards, principles and practices specified therein and, subject thereto, in accordance with GAAP and applicable Law as at the Balance Sheet Date, except as otherwise noted therein.
(b) The Financial Statements fairly present in all material respects the assets, liabilities, cash flow and financial condition and results of operations of the Company as of the times and for the periods referred to therein. Since the Balance Sheet Date, the Company has not made any material change in the accounting practices or policies applied in the preparation of the Financial Statements, except as required by applicable Law or GAAP.
(c) The Company and, to the Knowledge of the Company, any director, officer, employee, auditor, accountant or representative of the Company, has not received or otherwise had or obtained knowledge of any complaint, allegation, assertion or claim, whether written or, to the Knowledge of the Company, oral, regarding the accounting or auditing practices, procedures, methodologies or methods of the Company or their respective internal accounting controls, including any such complaint, allegation, assertion or claim that the Company has engaged in questionable accounting or auditing practices and there have been no internal investigations regarding accounting or revenue recognition discussed with, reviewed by or initiated at the direction of the chief executive officer, chief financial officer, general counsel, the board of directors of the Company or any committee thereof. The financial statements, when delivered by the Company for inclusion in the Information Statement for filing with the SEC following the date of this Agreement in accordance with Section 8.02, will comply in all material respects with the applicable accounting requirements and with the rules and regulations of the SEC and the Securities Act in effect as of such date.
Annex A-13
Section 4.06 Compliance with Laws.
(a) The Company is conducting and has conducted its business in compliance in all material respects with all Laws applicable to it and the Company’s business, properties or other assets.
(b) The Company has not received any written notice (official or otherwise) from any Governmental Authority (i) with respect to an alleged, actual or potential violation and/or failure to comply, in any material respect, with any such applicable Law or (ii) requiring the Company to take or omit any material action to ensure compliance with any such applicable Law.
(c) The Company possesses all permits, approvals, orders, authorizations, consents, licenses, certificates, franchises, accreditations, waivers, identification numbers, exemptions of, or filings or registrations (excluding Intellectual Property registrations and certifications) with, or issued by, any Governmental Authority necessary for the ownership and use of the assets of the Company and the operation of the Company’s business as currently conducted (the “Company Permits”), except where the failure to possess the same has not had or would not, individually or in the aggregate, reasonably be expected to be material to the Company. Except as has not had or would not, individually or in the aggregate, reasonably be expected to be material to the Company, all such Company Permits are valid and in full force and effect, and there are no lawsuits or other proceedings pending before or, to the Knowledge of the Company, threatened by any Governmental Authority that seek the revocation, cancellation, suspension or adverse material modification thereof. Except as has not had or would not, individually or in the aggregate, reasonably be expected to be material to the Company, the Company in not default, and, to the Knowledge of the Company, no condition exists that with notice or lapse of time or both would constitute a default, under the Company Permits.
Section 4.07 Absence of Certain Changes or Events. Since the Balance Sheet Date and through the date hereof, and except as expressly set forth on Schedule 4.07 or as required by this Agreement, (a) the Company has conducted its businesses in all material respects in the ordinary course (and in a manner consistent with past practice) and(b) there has not been any change, effect, event, circumstance, occurrence or state of facts that would, individually or in the aggregate, reasonably be expected to have a Company Material Adverse Effect.
Section 4.08 No Undisclosed Liabilities. Except (a) as disclosed, reflected or reserved against in the Financial Statements or the notes thereto, (b) for liabilities incurred in the ordinary course of business since the Balance Sheet Date, (c) as expressly permitted or contemplated by this Agreement or otherwise incurred in connection with the Transactions, (d) as disclosed on Schedule 4.08, (e) contingent liabilities under executory contracts and (f) for liabilities that have been discharged or paid in full in the ordinary course of business, as of the date hereof, the Company does not have any material liabilities of any nature, whether accrued, contingent or otherwise required to be reflected on a consolidated balance sheet prepared in accordance with GAAP consistently applied and in accordance with past practice.
Section 4.09 Information Supplied. The information supplied in writing by the Company for inclusion in the Information Statement will not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they were made, not false or misleading.
Section 4.10 Litigation.
(a) Except as set forth on Schedule 4.10(a), there is no material Action pending or, to the Knowledge of the Company, threatened against the Company or any of its Subsidiaries, or any property or asset of the Company or any of its Subsidiaries, that would, individually or in the aggregate, reasonably be expected to be material to the Company.
(b) Except as set forth on Schedule 4.10(b), the Company is not a party to or subject to the provisions of any outstanding Governmental Order (except if generally applicable without the Company being named therein) that would, individually or in the aggregate, reasonably be expected to be material to the Company.
Annex A-14
Section 4.11 Contracts.
(a) Schedule 4.11(a) sets forth a true and complete list as of the date hereof of the following types of Contracts to which the Company is a party or is bound (other than any Contracts under which the Company does not have any continuing or potential liability and the Lease Documents set forth on Schedule 4.18(c), Contracts set forth on Schedule 4.25, Company Benefit Plans set forth on Schedule 4.12(a)) (all such Contracts set forth on Schedule 4.11(a), or which are required to be so disclosed, the “Material Contracts”):
(i) each Contract with consideration paid or would reasonably be expected to be payable to the Company of more than $100,000, in the aggregate, over any twelve (12)-month period;
(ii) all distributor, agency, sales promotion, market research, marketing consulting and advertising Contracts or arrangements that are material to the business of the Company;
(iii) all Contracts (excluding Contracts for employment) with management and consultants;
(iv) all bonus and commission plans of the Company with a reasonably expected value in excess of $100,000 in any 12-month period;
(v) all Contracts involving the payment or payment of royalties or other amounts calculated based upon the revenues or income of the Company or income or revenues related to any Product of the Company to which the Company is a party;
(vi) all Contracts evidencing Indebtedness for borrowed money in an amount greater than $100,000, and any pledge agreements, security agreements or other collateral agreements in which the Company granted to any person a Lien on any of the property or assets of the Company;
(vii) all partnership, joint venture or similar agreement or arrangement, including as may be provided in any letter of intent, memorandum of understanding or agreement in principle;
(viii) all Contracts, including any grant agreements with any economic development corporation, with any Governmental Authority to which the Company is a party, other than any Company Permits;
(ix) all Contracts that (a) limit, or purport to limit, in any material respect, the ability of the Company to compete in any line of business or material business activity or with any Person or in any jurisdiction or during any period of time, excluding customary non-solicitation obligations entered into in the ordinary course of business and confidentiality agreements and agreements that contain customary confidentiality clauses, and (b) that impose “most favored nations” or “most favored supplier” restrictions;
(x) all Contracts that result in any Person or entity holding a power of attorney from the Company;
(xi) all leases or master leases of personal property reasonably likely to result in annual payments of $100,000 or more in a 12-month period;
(xii) any note, mortgage, indenture or other obligation or agreement or other instrument for or relating to indebtedness for borrowed money in excess of $100,000, or any guarantee of third party obligations in excess of $100,000, or any letters of credit, performance bonds or other credit support for the Company;
(xiii) all Contracts for the employment or engagement of any employee, officer, director or other individual service provider that (A) provide for annualized base compensation in excess of $200,000 or (B) are not terminable by the Company on no more than 30 days’ notice and without liability to or financial obligation by the Company;
(xiv) any collective bargaining agreement or other Contract with any labor union, works council, or other labor organization (each, a “CBA”);
Annex A-15
(xv) Contracts which involve the license or grant of rights under any Intellectual Property owned by a third party to the Company, or under Company Intellectual Property by the Company to a third party, excluding (A) nondisclosure agreements entered into in the ordinary course of business by the Company; (B) licenses of commercially available and/or off-the-shelf Software (including Software provided as a service) or other standard or commercially available Intellectual Property licensed under shrinkwrap, clickwrap, online terms of use or service or other standard license terms with an aggregate annual license cost of $100,000 or less; (C) Contracts between the Company and its customers entered into in the ordinary course of business in which Company Intellectual Property is licensed on a non-exclusive basis; (D) invention assignment and confidentiality agreements between the Company and its employees and/or independent contractors entered into by the Company in the ordinary course of business on the standard form(s) of such Contract(s) made available to Acquiror; (E) Contracts between the Company and its vendors or suppliers entered into in the ordinary course of business in which the Company has granted a license to the supplier or vendor (i) to use the Company’s trademarks, service marks, or other source identifiers for purposes of indicating that the Company is a customer of the vendor or supplier; (ii) to use feedback, suggestions or ideas provided by the Company to the vendor or supplier; or (iii) to use any Company Intellectual Property for purposes of providing goods or services to or as directed by the Company, and (F) Contracts under which the license or grant of rights is merely incidental to the transaction(s) contemplated by such Contract;
(xvi) any Contract that is a settlement, conciliation or similar agreement with any Governmental Authority or pursuant to which the Company will have any material outstanding obligation after the date of this Agreement;
(xvii) all Contracts for the development of Intellectual Property for the benefit of the Company (other than invention assignment and confidentiality agreements entered into with employees and contractors of the Company that have provisions relating to confidentiality and assignment of Intellectual Property that are materially similar to the confidentiality and Intellectual Property assignment provisions set forth in the standard form(s) of such agreement(s) used by the Company and made available to Acquiror); and
(xviii) any principal transaction Contract entered into in connection with a completed acquisition or disposition by the Company or any of its Subsidiaries involving consideration in excess of $250,000 of any Person or other business organization, division or business of any Person (including through merger or consolidation or the purchase of a controlling equity interest in or substantially all of the assets of such Person or by any other manner).
(b) Except as set forth on Schedule 4.11(b), the Company (i) is not, nor has it received written or, to the Knowledge of the Company, oral notice that any other party to any Material Contract is, except as such may be limited the Enforceability Exceptions, in material violation or material breach of or material default (immediately or upon notice or lapse of time) under or (ii) has not waived or failed to enforce any material rights or material benefits under any Material Contract to which it is a party or any of its properties or other assets is subject. No Material Contract is the subject of a written notice to terminate delivered or communicated in accordance with the terms of any Material Contract, except for any expiration of the term of a Material Contract following the date of this Agreement in accordance with its terms. Each Material Contract is in full force and effect and, subject to the Enforceability Exceptions, is legal, valid and binding on the Company, and, to the Knowledge of the Company, each other party thereto, except as would not be material and adverse to the Company, taken as a whole. Except as set forth on Schedule 4.11(b), there is no default under any such Material Contracts by the Company, or, to the Knowledge of the Company, any other party thereto, and no event has occurred that with the lapse of time or the giving of notice or both would constitute a default thereunder by the Company, or, to the Knowledge of the Company, any other party thereto, in each case, except as would not be material and adverse to the Company, taken as a whole.
Section 4.12 Employee Benefits.
(a) Schedule 4.12(a) sets forth an accurate and complete list of each material Company Benefit Plan.
(b) No Company Benefit Plan is or was at any time subject to the Laws of the United States nor any State therein, and no Company Benefit Plan benefits nor at any time benefited any individuals who are or were United States citizens and/or residents. The Company does not have any liability or obligation, contingent or actual, under ERISA or the Code either individually, collectively or as a result of any affiliation with an ERISA Affiliate.
Annex A-16
(c) With respect to each Company Benefit Plan, the Company has made available to Acquiror, if applicable (i) a true and complete copy of the plan document and all amendments thereto and each trust or other funding arrangement, (ii) copies of the most recent summary plan descriptions and summaries of material modifications, (iii) copies of annual reports and/or filings required to be made with any applicable Governmental Authority for the past three (3) plan years and (iv) any material non-routine correspondence from any Governmental Authority with respect to any Company Benefit Plan within the past three (3) years. The Company has no express commitment to modify, change or terminate any Plan, other than with respect to a modification, change or termination required by applicable Law.
(d) Neither the Company is, nor will it become obligated, whether under any Company Benefit Plan, employment agreement, consulting agreement, or other arrangement, whether written or unwritten (collectively, “Service Agreements”), to pay separation, severance, termination or similar benefits to any person directly as a result of the Transactions, nor will the Transactions accelerate the time of payment or vesting, or increase the amount, of any benefit or other compensation due to any individual. The Transaction shall not be the direct or indirect cause of any amount paid or payable by the Company being classified as an “excess parachute payment” under Section 280G of the Code.
(e) None of the Company Benefit Plans nor Service Agreements provides, nor does the Company have or reasonably expect to have any obligation to provide retiree medical to any current or former employee, officer, director or consultant of the Company after termination of employment or service except as may be required under applicable Law.
(f) Each Company Benefit Plan and each Service Agreement is and has been within the past six (6) years in compliance, in all material respects, in accordance with its terms and the requirements of all applicable Laws. The Company has performed, in all material respects, all obligations required to be performed by them under, are not in any material respect in default under or in violation of, and have no knowledge of any default or violation in any material respect by any party to, any Company Benefit Plan or Service Agreement. No Action is pending or, to the knowledge of the Company, threatened with respect to any Company Benefit Plan (other than claims for benefits in the ordinary course) or Service Agreement and, to the knowledge of the Company, no fact or event exists that could reasonably be expected to give rise to any such Action.
(g) There have been no acts or omissions by the Company that have given or could reasonably be expected to give rise to any material fines, penalties, Taxes or related charges under applicable Law for which the Company may be liable.
(h) All contributions, premiums or payments required to be made with respect to any Company Benefit Plan have been timely made to the extent due or properly accrued on the consolidated financial statements of the Company, except as would not result in material liability to the Company.
Section 4.13 Labor and Employment.
(a) Schedule 4.13(a) sets forth a true, correct and complete list of all current employees of TechyTrade, as of a date not more than five (5) days before the Closing Date, including any employee who is on a leave of absence of any nature, authorized or unauthorized, and sets forth for each such individual the following: (i) name; (ii) title or position (including whether full or part time); (iii) hire date; (iv) current annual base compensation rate; (v) commission, bonus or other incentive based compensation; and (vi) the term of their employment (indefinite or definite). As of the date hereof, all compensation, including wages, commissions and bonuses, due and payable to all employees for services performed on or prior to the date hereof, has been paid in full.
(b) Except as set forth on Schedule 4.13(b), (i) there are no material Actions pending or, to the knowledge of the Company, threatened in writing against the Company alleging violations of any Law pertaining to labor relations or employment matters, by any of their respective current or former employees, which Actions would be material to the Company, taken as a whole; (ii) the Company is not, nor has the Company been for the past two (2) years, a party to, bound by, or negotiating any collective bargaining agreement or other contract with a union, works council or labor organization applicable to persons employed by the Company, nor, to the knowledge of the Company, is there a union organizing campaign in progress with respect to any such employees; (iii) there are no unfair labor practice complaints pending against the Company before any Governmental Authority; and (iv) for the past two (2) years there has not been, nor, to the knowledge of the Company, has there been threatened in writing, any strike, slowdown, work stoppage, lockout, concerted refusal to work overtime or other material labor dispute affecting any employees of the Company.
Annex A-17
(c) The Company is, and for the last two (2) years has been, in compliance in all material respects with all applicable Laws relating to the employment, employment practices, employment discrimination, terms and conditions of employment, mass layoffs and plant closings (including the Worker Adjustment and Retraining Notification Act of 1988, as amended, or any similar Laws), immigration, meal and rest breaks, pay equity, workers’ compensation, family and medical leave, and occupational safety and health requirements, including those related to wages, hours, collective bargaining and the payment and withholding of Taxes and other sums as required by the appropriate Governmental Authority and are not liable for any arrears of wages, Taxes, penalties or other sums for failure to comply with any of the foregoing.
(d) To the Knowledge of the Company, no current or former employee or independent contractor of the Company is in any material respect in violation of any term of any employment agreement, nondisclosure agreement, common law nondisclosure obligation, fiduciary duty, noncompetition agreement, non-solicitation agreement, restrictive covenant or other obligation: (i) owed to the Company; or (ii) owed to any third party with respect to such Person’s right to be employed or engaged by the Company. To the Knowledge of the Company, no current employee of the Company with annualized base compensation at or above $250,000, has given notice to the Company that the employee intends to terminate his or her employment prior to the one-year anniversary of the Closing.
(e) The Company has promptly, thoroughly and impartially investigated all sexual harassment, or other unlawful discrimination or unlawful retaliation, complaints made by or against employees of the Company, in each case in connection with their employment with the Company, of which it has been made aware in the past two (2) years. With respect to each such complaint to the extent warranted based on the Company’s investigation, the Company has taken prompt corrective action that is reasonably calculated to prevent further improper action. The Company does not reasonably expect any material liabilities with respect to any such complaints and, to the Knowledge of the Company, there are no such complaints relating to officers, directors, employees, contractors, or agents of the Company relating to their employment with or service to the Company, that, if known to the public, would bring the Company into material disrepute.
(f) Except as would not result in material liability for the Company, in the past two (2) years the Company has fully and timely paid all (i) wages, salaries, wage premiums, commissions, overtime, bonuses, severance and termination payments, fees, and other compensation that has come due and payable to its current or former employees and independent contractors under applicable Laws, Contract or Company policy, and (ii) fines, Taxes, interest, or other penalties for any failure to pay or delinquency in paying such compensation.
Section 4.14 Taxes.
(a) The Company: (i) has duly and timely filed (taking into account any extension of time within which to file) all material Tax Returns required to be filed by any of them as of the date hereof and all such filed Tax Returns are complete and accurate in all material respects; (ii) has timely paid all material Taxes that are shown as due on such filed Tax Returns and any other material Taxes that the Company is otherwise obligated to pay, and no material penalties or charges are due with respect to the late filing of any Tax Return required to be filed by or with respect to any of them on or before the Effective Time; (iii) with respect to all material Tax Returns filed by or with respect to them, have not waived any statute of limitations with respect to Taxes or agreed to any extension of time with respect to a Tax assessment or deficiency; and (iv) do not have any deficiency, audit, examination, investigation or other proceeding in respect of Taxes or Tax matters pending or proposed or threatened in writing, for a Tax period which the statute of limitations for assessments remains open.
(b) The Company is not a party to, bound by or otherwise obligated under any Tax sharing agreement, Tax indemnification agreement, Tax allocation agreement or similar contract or arrangement (including any agreement, contract or arrangement providing for the sharing or ceding of credits or losses) or has a potential liability or obligation to any person as a result of or pursuant to any such agreement, contract, arrangement or commitment other than an agreement, contract, arrangement or commitment the primary purpose of which does not relate to Taxes.
(c) The Company will not be required to include any material item of income in, or exclude any material item of deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting for a taxable period ending on or prior to the Closing Date
Annex A-18
under Code Section 481(c) (or any corresponding or similar provision of state, local or non-U.S. income Tax Law); (ii) “closing agreement” as described in Code Section 7121 (or any corresponding or similar provision of state, local or non-U.S. income Tax Law) executed on or prior to the Closing Date; or (iii) installment sale made on or prior to the Closing Date.
(d) The Company has withheld and paid to the appropriate Tax authority all material Taxes required to have been withheld and paid in connection with amounts paid or owing to any current or former employee, independent contractor, creditor, stockholder or other third party and has complied in all material respects with all applicable Laws, rules and regulations relating to the payment and withholding of Taxes.
(e) The Company has not been a member of an affiliated group filing a consolidated, combined or unitary U.S. federal, state, local or non-U.S. income Tax Return (other than a group of which the Company was the common parent).
(f) The Company does not have material liability for the Taxes of any person (other than the Company) under Treasury Regulation Section 1.1502-6 (or any similar provision of state, local or non-U.S. Law), as a transferee or successor, by contract or otherwise.
(g) The Company does not have any request for a material ruling in respect of Taxes pending between the Company or any Company Subsidiary and any Tax authority.
(h) The Company has made available to Acquiror true, correct, and complete copies of the income Tax Returns filed by the Company for tax years through 2025.
(i) The Company has not in any year for which the applicable statute of limitations remains open distributed stock of another person, or has had its stock distributed by another person, in a transaction that was purported or intended to be governed in whole or in part by Section 355 or Section 361 of the Code.
(j) The Company has not engaged in or entered into a “listed transaction” within the meaning of Treasury Regulation Section 1.6011-4(b)(2) (or any corresponding or similar provision of state, local or non-U.S. income Tax Law).
(k) No Governmental Authority has asserted in writing or, to the knowledge of the Company, has threatened to assert against the Company any deficiency or claim for any Taxes or interest thereon or penalties in connection therewith.
(l) There are no Tax Liens upon any assets of the Company except for Permitted Liens.
(m) The Company has not been a United States real property holding corporation within the meaning of Section 897(c)(2) of the Code during the applicable period specified in Section 897(c)(1)(A)(ii) of the Code. The Company: (A) is not a “controlled foreign corporation” as defined in Section 957 of the Code, (B) does not have a permanent establishment (within the meaning of an applicable Tax treaty) or otherwise has an office or fixed place of business in a country other than the country in which it is organized or (C) is not otherwise subject to taxation in a country other than the country in which it is organized.
(n) The Company is in compliance in all material respects with applicable transfer pricing Laws.
Section 4.15 Intellectual Property.
(a) Schedule 4.15(a) contains a complete and accurate list of all (i) issued patents and pending patent applications, (ii) trademark and service mark registrations and applications, (iii) copyright registrations and (iv) registered domain names, in each case that are owned by the Company (collectively, “Registered IP”), indicating for each item, as applicable, the registration or application number, the applicable filing jurisdiction and the date of filing or issuance, and registrar. The Company exclusively owns all right, title, and interest in and to the Registered IP, free and clear of any Liens other than Permitted Liens. To the Knowledge of the Company, the Registered IP is subsisting and, excluding any Registered IP which is the subject of an application for registration or issuance, has not been held invalid by any Governmental Authority and is enforceable, in each case, except as would not be material and adverse to the Company, taken as a whole.
(b) The Company solely and exclusively owns all right, title, and interest in and to or is licensed to use or otherwise has the right to use all Intellectual Property used in or necessary to the conduct of the business of the Company as currently conducted, except as would not be material and adverse to the Company, taken as a whole.
Annex A-19
All Owned Intellectual Property and Intellectual Property licensed to the Company by a third party that is used in or necessary to the conduct of the business of the Company as currently conducted shall be owned or available for use by the Company immediately after the Closing on terms and conditions substantially the same as those under which any Company owned or used such Intellectual Property immediately prior to the Closing, in each case, except as would not be material and adverse to the Company, taken as a whole.
(c) Except as set forth on Schedule 4.15(c), to the Knowledge of the Company (i) the operation of the business of the Company as currently conducted does not infringe, misappropriate, dilute or otherwise violate any third-party Intellectual Property, except as would not be material and adverse to the Company, taken as a whole, and (ii) no third party infringes, misappropriates, dilutes or otherwise violates on the date of this Agreement, and no third party has infringed, misappropriated, diluted or otherwise violated any material Owned Intellectual Property.
(d) As of the date hereof, there is no Action pending or, to the Knowledge of the Company, threatened in writing against the Company (i) challenging the ownership, validity, registrability, patentability, or enforceability of the Owned Intellectual Property (excluding office actions and similar ex-parte proceedings in connection with the prosecution of applications for the registration or issuance of any Intellectual Property) or (ii) asserting that the Company has infringed, misappropriated, diluted or otherwise violated any third-party Intellectual Property, in the case of each of clause (i) and (ii), except as would not be material and adverse to the Company, taken as a whole.
(e) Intentionally Omitted.
(f) The Company has not received any funding of any university or other educational or research center or Governmental Authority. No such university, educational or research center, or Governmental Authority has any rights in or to any Owned Intellectual Property or, to the Knowledge of Company, any other Intellectual Property used in or necessary for the business of the Company as currently conducted.
(g) To the Knowledge of the Company, none of the Company Software is developed, used, distributed or modified under any Open Source Software license in a manner which has or would require any disclosure, licensing or distribution of the source code of any such Company Software to any Person, other than the applicable Open Source Software. To the Knowledge of the Company, the Company has complied, and currently complies, in all material respects with the terms of all applicable Open Source Software licenses.
(h) The Company has taken and takes commercially reasonable actions to maintain, protect and enforce Intellectual Property rights in the trade secrets owned by the Company.
Section 4.16 Data Protection.
(a) The Company (i) has been in compliance in all material respects with all Privacy Laws and (ii) has not been subject to any regulatory audits or, to the Knowledge of the Company, investigations by any Governmental Authority relating to Privacy Laws. The Company has taken commercially reasonable steps to ensure that all Personal Information is protected in all material respects against loss and against unauthorized access, use, modification, disclosure or other use or misuse. To the Knowledge of the Company, there has been no loss, theft or unauthorized access to or misuse of any Personal Information, in each case, that has resulted in, or is reasonably likely to result in, material liability to the Company, taken as a whole.
(b) The Company has not received any written requests, complaints or objections to its collection or use of Personal Information from any data protection authority or third party (including data subjects) that remains unresolved that has resulted in, or is reasonably likely to result in, material liability to the Company, taken as a whole. To the Knowledge of the Company, no individual has been awarded compensation from the Company under any Privacy Laws, and no written claim for such compensation is outstanding.
(c) The Company does not sell, rent or otherwise make available to any Person any Personal Information, except in a manner that complies in all material respects with the applicable Privacy Laws. The execution, delivery and performance of this Agreement and the transactions contemplated herein comply, and will comply, in all material respects, with all Privacy Laws and other contractual commitments related to the privacy and security of Personal Information to which the Company is bound, except as would not be material and adverse to the Company, taken as a whole.
Annex A-20
Section 4.17 Information Technology.
(a) The IT Systems: (i) operate and perform in material accordance with the requirements of the Company for the operation of its business as currently conducted and (ii) to the Knowledge of the Company, are free from bugs and other defects, in each case, except as would not be material and adverse to the Company, taken as a whole.
(b) The Company uses commercially reasonable efforts to protect the confidentiality, integrity and security of the IT Systems used in the operation of the business of the Company from any unauthorized use, access, interruption, or modification. Such IT Systems are sufficient for current needs of the Company, including as to capacity, scalability and ability to process current and anticipated peak volumes in a timely manner. The IT Systems include a sufficient number of license seats for all Software licensed by the Company from third parties as necessary for the usage of such Software in the operation of the business of the Company as currently conducted.
(c) To the Knowledge of the Company, there have been no unauthorized intrusions, failures, breakdowns, security breaches, continued substandard performance, or other adverse events affecting any such IT Systems that have caused any substantial disruption of or interruption in or to the use of such IT Systems or any unauthorized use, misappropriation, modification, encryption, corruption, disclosure, or transfer of any information or data contained therein, in each case, that has resulted in, or is reasonably likely to result in, material liability to the Company. The Company maintains commercially reasonable disaster recovery and business continuity plans, procedures and facilities in connection with the operation of the business of the Company, acts in compliance therewith, and has taken commercially reasonable steps to test such plans and procedures on a periodic basis, and such plans and procedures have been proven effective upon such testing in all material respects.
Section 4.18 Real Property.
(a) The Company does not own any real property.
(b) Schedule 4.18(b) contains a complete and accurate list by property, city, state and country, of all real property leasehold or subleasehold estates and other rights to possess or occupy any land, buildings, structures, improvements, fixtures or other interest in real property held by the Company as of the date of this Agreement (the “Leased Company Properties”). The Company is the sole legal and beneficial owner of a leasehold or subleasehold interest in, or other right to possess or occupy, the Leased Company Properties.
(c) Schedule 4.18(c) contains a complete and accurate list of all leases, subleases, licenses, concessions, and other Contracts, agreements and leasehold arrangements and all related supplemental documents (collectively, the “Lease Documents”) pursuant to which the Company leases, licenses, subleases or otherwise occupies any Leased Company Property on the date hereof. The Company has delivered to Acquiror a true and complete copy of each such Lease Document. Neither the Company nor, to the Knowledge of the Company, any other party to any Lease Document is in material breach or material default under such Lease Document, and no event has occurred or circumstances exist which, with the delivery of notice, the passage of time or both, would constitute such a breach or default, or permit the termination or acceleration of rent under such Lease Document, by the Company or, to the Knowledge of the Company, any other party thereto.
(d) Each Lease Document is a written agreement in full force and effect, and, subject to the Enforceability Exceptions, is legal, valid, binding and enforceable against the Company that is a party to such Lease Document and, to the Knowledge of the Company, any other party to such Lease Document. The Company has paid the rent and all other sums that are due and payable under such Lease Documents and there are no significant arrears thereunder due and payable by the Company.
(e) To the Knowledge of the Company, there exist no restrictions, covenants or encumbrances which encumber any of the Leased Company Properties and which prevent any of the Leased Company Properties from being used now or in the future for their current use or would prevent, or require consent from a third party as a result of, the consummation of the transactions contemplated by this Agreement or which would be material and adverse to the Company, taken as a whole.
(f) The Company has not, at any time, given any covenant or entered into any agreement in respect of any leasehold real property other than the Leased Company Properties in respect of which any material contingent liability of the Company remains as of the date of this Agreement. The Company has not subleased, licensed or otherwise granted any Person the right to use or occupy any Leased Company Property or any portion thereof, and the Company has not collaterally assigned or granted any other security interest in any Lease Document or any interest therein.
Annex A-21
(g) As of the date of this Agreement, to the Knowledge of the Company, there are no material outstanding Actions to which the Company is a party in respect of any of the Leased Company Properties, other than nondelinquent real property assessments affecting the Leased Company Properties. As of the date of this Agreement, the Company’s possession and quiet enjoyment of the Leased Company Property under each Lease Document is not materially disturbed.
Section 4.19 Anti-Bribery; Trade Controls Compliance.
(a) Anti-Bribery. The Company and each of its managers, officers, directors, employees, and to the Knowledge of the Company, agents, and any other Person acting on their behalf, (i) are and have been, in compliance with the anti-bribery Laws and anti-corruption Laws of each jurisdiction in which the Company operates or has operated, including the U.S. Foreign Corrupt Practices Act of 1977, as amended (collectively, “Anti-Bribery Laws”), and (ii) have not paid, given, offered or promised to pay, or authorized or ratified the payment or transfer, directly or indirectly, of any monies or anything of value to any Public Official or other Person, for the purpose of corruptly influencing any act or decision of such Public Official or of a Governmental Authority, or any other Person, to obtain or retain business, to direct business to any Person, or to secure any other improper benefit or advantage. Except as set forth on Schedule 4.19(a), the Company is not subject, and has not been subject, to any Actions or made any disclosures, voluntary or otherwise, to any Governmental Authority relating to the Anti-Bribery Laws.
(b) Trade Control Compliance. To the Knowledge of the Company, the Company is and has been, in compliance in all respects with all applicable international trade control compliance Laws, including but not limited to: (a) U.S. Laws governing economic sanctions, including those administered by the U.S. Treasury Department’s Office of Foreign Assets Control codified at 31 C.F.R. Part 500 et. seq., and the U.S. Department of State (“Sanctions”); (b) U.S. Laws governing the exportation of goods, technology, software, and services, including the Export Administration Regulations (15 C.F.R. § 730 et seq.), and the International Traffic in Arms Regulations (22 C.F.R. § 120 et seq.); (c) U.S. Laws governing the importation of goods, including laws administered by U.S. Customs and Border Protection; and (d) U.S. Laws governing international boycotts administered by the U.S. Department of Commerce and the Internal Revenue Service (collectively, the “International Trade Laws”). None of the Company and its directors or director equivalents, members, officers, employees, or to the Knowledge of the Company, agents, representatives or other Persons acting on behalf of the Company, (a) have been the target of Sanctions, (b) located, organized, or ordinarily resident in a jurisdiction subject to comprehensive Sanctions (as of the date of this Agreement, Cuba, Iran, North Korea, Syria, and the Crimea, so-called Donetsk People’s Republic, and so-called Luhansk People’s Republic regions of Ukraine) (“Embargoed Jurisdiction”), or (c) owned fifty percent (50%) or more, directly or indirectly, individually or in aggregate, by Persons listed in (a) or (b) (collectively, a “Sanctioned Person”). To the Knowledge of the Company, the Company has not engaged in any unlawful dealings or transactions, directly or indirectly, with any Sanctioned Persons. The Company is not subject, and have not been subject to any Actions, or made any disclosures to any Governmental Authority, involving the Company relating to the International Trade Laws. The Company has implemented and administered internal controls, policies and procedures designed, that are reasonably designed to promote compliance with International Trade Laws.
Section 4.20 Insurance.
(a) Schedule 4.20(a) sets forth a true and complete list of the material current insurance policies or binders maintained by the Company (the “Insurance Policies”). To the Knowledge of the Company, there are no events, circumstances or other liabilities that would reasonably be expected to give rise to a material claim under the Insurance Policies.
(b) Except as has not had or would not, individually or in the aggregate, reasonably be expected to be material to the Company, the Insurance Policies are in full force and effect as of the date of this Agreement with respect to the Company, and the limits thereunder have not been impaired, exhausted or materially diminished.
(c) As of the date hereof, the Company has not received any written or oral notice of cancellation of, a material premium increase (relative to others in the industry in which the Company operates) with respect to, or of a material alteration of coverage under, any Insurance Policy. Except as has not had or would not, individually or in the aggregate, reasonably be expected to be material to the Company, all of the Insurance Policies (i) are valid and binding in accordance with their terms, subject to Enforceability Exceptions and (ii) have not been subject to any lapse in coverage. There are no material claims related to the Company or the assets, business, operations, employees, officers and directors of the Company pending under any such Insurance Policies as to which coverage has been denied or disputed or in respect of which there is an outstanding reservation of rights.
Annex A-22
Section 4.21 Competition Regulation. Except as set forth on Schedule 4.21, the Company is in compliance with all applicable Antitrust Laws in all material respects. The Company is not a party to any agreement or arrangement with a Governmental Authority under any Antitrust Laws in any jurisdiction in which the Company has assets or carries on or intends to carry on business.
Section 4.22 Environmental Matters. Except as has not had or would not, individually or in the aggregate, reasonably be expected to be material to the Company:
(a) the Company is in material compliance in all respects with all Environmental Laws and all material Company Permits required under Environmental Laws in connection with the operation of the Company’s business or ownership or operation of the Leased Company Properties, which Company Permits have been obtained by the Company and are current and valid, except as such Company Permit would not be material to the Company’s business, taken as a whole;
(b) there are no Actions or Governmental Orders pending, or to the Knowledge of the Company, threatened, against the Company, nor, to the Knowledge of the Company, has the Company received any written notification of or otherwise been made aware of, any actual or alleged violation of, or liability under, Environmental Laws;
(c) the Company (or to the Knowledge of the Company, any other Person to the extent giving rise to liability for the Company) has not manufactured, generated, treated, stored, disposed or arranged for disposal of, transported, released, exposed any Person to, or owned or operated any property or facility contaminated by, any Hazardous Material under circumstances or in quantities that violate Environmental Laws or which would reasonably be expected to give rise to liability for the Company pursuant to Environmental Laws; and
(d) the Company has furnished to the Acquiror copies of all material environmental reports, assessments and audits in its possession or reasonable control relating to the Company’s compliance with Environmental Laws or the environmental condition of the real property operated or leased by the Company in connection with its business.
Section 4.23 Customers and Suppliers.
(a) Schedule 4.23(a) sets forth a true, correct and complete list, as of the date of this Agreement, of the 10 largest customers of the Company (each, a “Material Customer”), the year ended March 31, 2025, measured by the amount of revenue received by the Company during such period. Except as set forth on Schedule 4.23(a), as of the date hereof, the Company has not since the Balance Sheet Date received any written, or to the Knowledge of the Company, oral notice that any Material Customer has cancelled, materially decreased or otherwise materially modified, or intends to cancel, materially decrease or otherwise materially modify, its relationship with the Company.
(b) Schedule 4.23(b) sets forth a complete and correct list, as of the date of this Agreement, of the 10 largest vendors, suppliers, third-party service providers and other similar business relations of the Company (each, a “Material Vendor”) during the year ended March 31, 2025, measured by the amount of expenditure by the Company during such period. Except as set forth in Schedule 4.23(b), the Company has not as of the Balance Sheet Date received any written, or to the Knowledge of the Company, oral notice that any Material Vendor has cancelled, terminated or otherwise materially modified, or intends to cancel, terminate or otherwise materially modify its relationship with the Company.
Section 4.24 Brokers. Except as set forth on Schedule 4.24, no broker, investment banker, financial advisor or other Person, the fees and expenses of which will be paid by the Company pursuant to an engagement letter entered into therewith, is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission in connection with the Transactions based upon arrangements made by or on behalf of the Company.
Section 4.25 Affiliate Agreements. Except as set forth on Schedule 4.25, the Company is not party to any transaction, agreement, arrangement or understanding with any (a) present or former executive officer or director of the Company, (b) beneficial owner (within the meaning of Section 13(d) of the Exchange Act) of five (5%) percent or more of the capital stock or equity interests of the Acquiror, Merger Sub or the Company or (c) Affiliate, “associate” or member of the “immediate family” (as such terms are respectively defined in Rules 12b-2 and 16a-1 of the Exchange Act) of any of the foregoing.
Annex A-23
Section 4.26 Assets. The Company (i) owns or controls 1,000 bitcoin (the “Bitcoin”) in TechyTrade’s wholly-owned subsidiary, TechyTrade FZ LLC, and the Bitcoin are identifiable, segregated and held free and clear of any encumbrances or restrictions of any kind and (ii) has no indebtedness other than trade payables, and such trade payables were less than fifty percent (50%) of its cash on and in bank accounts; and (iii) the Bitcoin are maintained within a segregated sub-wallet structure created and administered by MindWave Ltd. (“MindWave”) for the benefit of the Company, with the private keys, full beneficial ownership and control of the segregated wallet(s) are held by and shall remain under the custody and ownership of TechyTrade FZ LLC, and (iv) the share of TechyTrade were duly issued to Calfin Capital Private Limited (“Calfin”) as consideration for the acquisition of those Bitcoin under the share subscription letter, dated November 14, 2025, by and between TechyTrade and Calfin. This representation is referred to herein as the “Asset Representation.” Simultaneously with the entry into this Agreement, the Company shall provide an attestation letter, dated as of the Closing Date, prepared by the Company’s independent registered public accounting firm evidencing the ownership of the Bitcoin in connection with the Asset Representation.
Section 4.27 No Other Representations or Warranties. The representations and warranties made by the Company in this Article IV are the exclusive representations and warranties made by the Company, its Affiliates and their respective Representatives. Except for the representations and warranties contained in this Article IV, neither the Company nor any other Person has made or makes any other express or implied representation or warranty, either written or oral, on behalf of the Company, to the accuracy or completeness of any information regarding the Company available to the other parties or their respective Representatives and expressly disclaims any such other representations or warranties. For the avoidance of doubt, each of the Company, its Affiliates and each of their respective Representatives has not made and does not make any express or implied representation or warranty, either written or oral, with respect to the Company. In particular, without limiting the foregoing, neither the Company nor any other Person makes or has made any representation or warranty to the other parties hereto, and shall have no liability in respect of, with respect to (a) any financial projection, forecast, estimate, budget or prospect information relating to the Company or (b) any oral or, except for the representations and warranties expressly made by the Company in this Article IV written information made available to the other parties hereto in the course of their evaluation of the Company and the negotiation of this Agreement or in the course of the Transactions. The Company hereby acknowledges and agrees with the statements and provisions set forth in Section 5.18.
Article V
REPRESENTATIONS AND WARRANTIES OF ACQUIROR AND MERGER SUB
Except as set forth in the Acquiror Disclosure Schedules (each of which qualifies (a) the correspondingly numbered representation, warranty or covenant if specified therein and (b) such other representations, warranties or covenants where its relevance as an exception to (or disclosure for purposes of) such other representation, warranty or covenant is reasonably apparent on its face) or in the Acquiror SEC Reports filed or furnished by Acquiror on or before the date of this Agreement (excluding (i) any disclosures in such Acquiror SEC Reports under the headings “Risk Factors” or “Forward-Looking Statements” and other disclosures that are predictive, cautionary or forward looking in nature and (ii) any exhibits or other documents appended thereto), each of Acquiror and Merger Sub, jointly and severally, represents and warrants to the Company as follows:
Section 5.01 Organization, Standing and Corporate Power.
(a) Each of Acquiror and Merger Sub is a corporation duly incorporated, validly existing and in good standing under the Laws of its jurisdiction of formation and has all requisite corporate power and authority to carry on its business as now being conducted. Merger Sub has no assets or operations other than those required to effect the Transactions contemplated hereby. Acquiror is duly qualified or licensed to do business and is in good standing in each jurisdiction in which the conduct of its business or the ownership, leasing or operation of its properties makes such qualification or licensing necessary, except as would not have an Acquiror Material Adverse Effect.
(b) Merger Sub is a corporation duly organized, validly existing and in good standing under the Law of the State of Delaware, with full corporate power and authority to enter into this Agreement and perform its obligations hereunder. Other than Merger Sub, Acquiror has no other Subsidiaries or any equity or other interests in any other Person.
Annex A-24
(c) Acquiror has provided to the Company a true, complete and correct copy of the Acquiror Organizational Documents and the certificate of incorporation and bylaws of Merger Sub and there are no other Contracts which would amend, supplement or relate to the subject matters described in the Acquiror Organizational Documents or the certificate of incorporation and bylaws of Merger Sub, provided however, that the Acquiror will file the Certificate of Amendment with the Secretary of State of the State of Delaware to amend the Acquiror Charter following the Action Effective Time to implement the Reverse Stock Split.
Section 5.02 Corporate Authority; Approval; Non-Contravention; Government Approvals.
(a) Each of Acquiror and Merger Sub has the requisite corporate power and authority and has taken all corporate action necessary in order to execute, deliver and perform its obligations under this Agreement and the Acquiror Support Agreement, as applicable, and subject to satisfaction of the conditions to Closing contemplated hereby, to consummate the Transactions. The execution, delivery and performance by Acquiror and Merger Sub of this Agreement and the Acquiror Support Agreement, as applicable, and the consummation by it of the Transactions, have been duly and validly authorized by all necessary corporate consent and authorizations on the part of Acquiror and Merger Sub, and no other corporate or other actions on the part of Acquiror or Merger Sub are necessary to authorize the execution and delivery by Acquiror or Merger Sub of this Agreement, the Acquiror Support Agreement, as applicable, and the consummation by it of the Transactions, in each case, subject to receipt of the Acquiror Stockholder Approvals. This Agreement has been duly executed and delivered by Acquiror and Merger Sub and, assuming due authorization, execution and delivery hereof by the other parties, is a legal, valid and binding obligation of Acquiror and Merger Sub, enforceable against Acquiror and Merger Sub in accordance with its terms (subject to the Enforceability Exceptions).
(b) The execution, delivery, and performance of this Agreement and the Acquiror Support Agreement, as applicable, and the consummation of the Transactions, and (in the case of Acquiror) subject to receipt of the Acquiror Stockholder Approvals, do not, and will not, constitute or result in (i) a breach or violation of, or a default under, the Acquiror Organizational Documents or any organizational documents of Merger Sub or (ii) with or without notice, lapse of time or both, a breach or violation of, a termination (or right of termination) of or default under, the creation or acceleration of any obligations under or the creation of a Lien on any of the assets of Acquiror, Merger Sub or any of their Affiliates pursuant to, any Contract to which Acquiror, Merger Sub or any of their Affiliates is a party or, assuming (solely with respect to performance of this Agreement and consummation of the Transactions) compliance with the matters referred to in Section 5.02(a), under any Law to which Acquiror, Merger Sub or any of their Affiliates is subject, except (in the case of clause (ii) above) for such violations, breaches or defaults which has not had or would not, individually or in the aggregate, reasonably be expected to materially impair, delay or prohibit the ability of Acquiror or Merger Sub to enter into, perform its obligations under this Agreement and consummate the Transactions.
(c) No consent of, or registration, declaration, notice or filing with, any Governmental Authority is required by or with respect to Acquiror or Merger Sub in connection with the execution and delivery by Acquiror or Merger Sub of this Agreement or the consummation of the Transactions contemplated by this Agreement or the Acquiror Support Agreement, except for (i) the filing with the SEC of (A) the Information Statement and (B) such reports under Section 13(a) or 15(d) of the Exchange Act as may be required in connection with this Agreement, the Acquiror Support Agreement or the transactions contemplated hereby or thereby, (ii) filing of the Certificate of Designation, (iii) filing of the Certificate of Amendment, (iv) filing of the Certificate of Merger or (vi) such other consents, registrations, declarations, notices and filings which, if not obtained or made, would not have an Acquiror Material Adverse Effect.
Section 5.03 Compliance with Laws. Acquiror and Merger Sub are, and since their respective dates of incorporation, have been, operating in all material respects in a manner that is customary for businesses similar to Acquiror and Merger Sub, and each of Acquiror and Merger Sub is conducting and, since their respective dates of incorporation, has conducted its business in material compliance with all Laws, and no notices have been received by either Acquiror or Merger Sub from any Governmental Authority or any other Person alleging an uncured material violation of any Law.
Section 5.04 Employee Benefit Plans. Except as may be contemplated by the 2024 Plan and the Acquiror Equity Plan Proposal, neither Acquiror nor Merger Sub maintains or contributes to any Benefit Plan. Neither the execution and delivery of this Agreement nor the consummation of the transactions contemplated by this Agreement (either alone or in combination with another event) will (i) result in any payment (including severance, unemployment compensation, golden parachute, bonus or otherwise) becoming due to any shareholder, stockholder, director, officer
Annex A-25
or employee of Acquiror or Merger Sub, or (ii) result in the acceleration, vesting or creation of any rights of any shareholder, director, officer or employee of Acquiror or Merger Sub to payments or benefits or increases in any existing payments or benefits or any loan forgiveness.
Section 5.05 Indebtedness. As of the date hereof, Acquiror does not have, or have any present intention, agreement, arrangement or understanding to enter into or incur, any obligations with respect to or under any Indebtedness.
Section 5.06 Taxes.
(a) Each of Acquiror and Merger Sub is and has at all times since its date of formation been, treated as a corporation for U.S. federal income tax purposes.
(b) Each of Acquiror and Merger Sub has timely filed with the appropriate Tax Authority, or has caused to be timely filed on its behalf (taking into account any valid extension of time within which to file), all material Tax Returns required to be filed by it, and all such Tax Returns were and are true, correct and complete in all material respects and were prepared in compliance in all material respects with all applicable Laws. Each of Acquiror and Merger Sub has timely paid all material amounts of Taxes due and payable (whether or not shown on any Tax Return), other than Taxes being contested in good faith and for which adequate reserves have been established in accordance with GAAP.
(c) Each of Acquiror and Merger Sub, as applicable, has complied in all material respects with all applicable Laws relating to the payment and withholding of Taxes and Tax information reporting, collection and retention and has, within the time and in the manner prescribed by applicable Laws, (i) withheld all material amounts of Taxes required to have been withheld by it in connection with amounts paid to any employee, independent contractor, creditor, stockholder or any other third party, and (ii) timely remitted such amounts required to have been remitted to the appropriate Tax Authority.
(d) No claim, assessment, deficiency or proposed adjustment for any Tax has been asserted or assessed by any Tax Authority against Acquiror or Merger Sub that remains unresolved or unpaid except for claims, assessments, deficiencies or proposed adjustments being contested in good faith and for which adequate reserves have been established in accordance with GAAP.
(e) There is no Tax audit, examination or other Action of Acquiror or Merger Sub presently in progress, and there are no waivers, extensions or requests for any waivers or extensions of any statute of limitations currently in effect with respect to any material Taxes of Acquiror or Merger Sub.
(f) Neither Acquiror nor Merger Sub is or has been (i) a party to any Tax sharing, indemnification, allocation or similar agreement or arrangement (excluding any commercial contract entered into in the ordinary course of business and not primarily related to Taxes), (ii) a member of an affiliated, consolidated, combined, unitary or similar Tax group (other than any such Tax group the common parent of which was the Company), or (iii) a party to any “listed transaction” under Treasury Regulations Section 1.6011-4(b) (2) (or any similar or corresponding provision of U.S. state or local or non-U.S. Law).
(g) Acquiror and Merger Sub do not have any liability for Taxes of any other Person as a result of Treasury Regulations Section 1.1502-6 (or any similar provision of U.S. state or local or non-U.S. Law), as a transferee or successor, or by operation of Law.
(h) Acquiror and Merger Sub will not be required to include any material item of income in, or exclude any material deduction from, taxable income for any taxable period (or portion thereof) ending after the Closing Date as a result of any: (i) change in method of accounting, or use of an improper method of accounting, for a taxable period (or portion thereof) ending on or prior to the Closing Date; (ii) “closing agreement” as described in Section 7121 of the Code (or any corresponding or similar provision of U.S. state or local or non-U.S. Law) executed on or prior to the Closing Date; (iii) installment sale or open transaction disposition made on or prior to the Closing Date; (iv) prepaid amount received or deferred revenue accrued on or prior to the Closing Date outside of the ordinary course of business; or (v) intercompany item under Treasury Regulation Section 1.1502-13 (or any corresponding or similar provision of U.S. state or local or non-U.S. Law) or excess loss account under Treasury Regulation Section 1.1502-19 (or any corresponding or similar provision of U.S. state or local or non-U.S. Law).
Annex A-26
(i) There are no Liens for Taxes on any assets of either Acquiror or Merger Sub other than Permitted Liens.
(j) No written claims have ever been made by any Tax Authority in a jurisdiction where Acquiror and Merger Sub do not file Tax Returns that the Company is or may be subject to taxation by that jurisdiction, which claims have not been resolved or withdrawn.
(k) Neither Acquiror or Merger Sub has been either a “distributing corporation” or a “controlled corporation” within the respective meanings of such terms under Code Section 355(a)(1)(A) in a distribution of stock qualifying under Code Section 355 (i) in the two years before the date of this Agreement or (ii) in a distribution that could otherwise constitute part of a “plan” or “series of related transactions” within the meaning of Code Section 355(e) in conjunction with the Transactions.
(l) The Acquiror is not and has never been a “United States real property holding corporation” within the meaning of Code Section 897(c)(2).
(m) The Merger Sub has been formed solely for the purpose of effecting the transactions contemplated under this Agreement and has not engaged in any activity other than activity consistent with this purpose.
(n) Neither Acquiror nor Merger Sub has taken or agreed to take any action not contemplated by this Agreement or the Acquiror Support Agreement that would reasonably be expected to prevent the Transactions from qualifying for the Intended Tax Treatment.
(o) To the Knowledge of Acquiror, no facts or circumstances exist that would reasonably be expected to prevent the Transactions from qualifying for the Intended Tax Treatment.
Section 5.07 Brokers. No broker, investment banker, financial advisor or other Person, other than those set out in Schedule 5.07, the fees and expenses of which will be paid by Acquiror or Merger Sub pursuant to an engagement letter entered into therewith, is entitled to any broker’s, finder’s, financial advisor’s or other similar fee or commission in connection with the Transactions based upon arrangements made by or on behalf of Acquiror, Merger Sub or any of their Affiliates.
Section 5.08 Acquiror SEC Reports; Financial Statements; Sarbanes-Oxley Act.
(a) Acquiror has filed or furnished in a timely manner all required registration statements, reports, schedules, forms, statements and other documents required to be filed or furnished by it with the SEC since March 3, 2025, pursuant to the Exchange Act or the Securities Act (collectively, as they have been amended since the time of their filing and including all exhibits thereto, the “Acquiror SEC Reports”). None of the Acquiror SEC Reports, as of their respective dates (or if amended or superseded by a filing prior to the date of this Agreement or the Closing Date, then on the date of such filing), contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements made therein, in light of the circumstances under which they were made, not misleading. The audited financial statements and unaudited interim financial statements (including, in each case, the notes and schedules thereto) included in the Acquiror SEC Reports complied as to form in all material respects with the published rules and regulations of the SEC with respect thereto, were prepared in accordance with GAAP applied on a consistent basis during the periods involved (except as may be indicated therein or in the notes thereto and except with respect to unaudited statements as permitted by Form 10-Q of the SEC), and fairly present (subject, in the case of the unaudited interim financial statements included therein, to normal year-end adjustments and the absence of complete footnotes) in all material respects the financial position of Acquiror as of the respective dates thereof and the results of their operations and cash flows for the respective periods then ended.
(b) Acquiror has established and maintains disclosure controls and procedures (as defined in Rule 13a-15 under the Exchange Act). Such disclosure controls and procedures are designed to ensure that material information relating to Acquiror and other material information required to be disclosed by Acquiror in the reports and other documents that it files or furnishes under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC, and that all such material information is accumulated and communicated to Acquiror’s management, including its principal executive officer and its principal financial officer as appropriate to allow timely decisions regarding required disclosure and to make the certifications required pursuant to Sections 302 and 906 of the Sarbanes-Oxley Act. Such disclosure controls and procedures are effective in timely alerting Acquiror’s principal executive officer and principal financial officer to material information required to be included in Acquiror’s periodic reports required under the Exchange Act.
Annex A-27
(c) Except as disclosed in the Acquiror SEC Reports, Acquiror has established and maintained a system of internal control over financial reporting (as defined in Rule 13a-15 under the Exchange Act). Such internal control over financial reporting is sufficient to provide reasonable assurance regarding the reliability of Acquiror’s financial reporting and the preparation of Acquiror’s financial statements for external purposes in accordance with GAAP.
(d) There are no outstanding loans or other extensions of credit made by Acquiror to any executive officer (as defined in Rule 3b-7 under the Exchange Act) or director of Acquiror. Acquiror has not taken any action prohibited by Section 402 of the Sarbanes-Oxley Act.
(e) Neither Acquiror (including any employee thereof) nor Acquiror’s independent auditors has identified or been made aware of (i) any significant deficiency or material weakness in Acquiror’s internal control over financial reporting, (ii) any fraud, whether or not material, that involves Acquiror’s management or other employees who have a role in the preparation of financial statements or Acquiror’s internal control over financial reporting or (iii) any claim or allegation regarding any of the foregoing.
(f) Acquiror does not have any past due liability relating to the PCAOB issuer accounting support fee.
(g) As of the date hereof, there are no outstanding comments from the SEC with respect to the Acquiror SEC Reports. To the Knowledge of Acquiror, none of the Acquiror SEC Reports filed on or prior to the date hereof is subject to ongoing SEC review or investigation as of the date hereof.
Section 5.09 Business Activities; Absence of Changes.
(a) There is no agreement, commitment or Governmental Order binding upon Acquiror or to which Acquiror is a party which has had or would reasonably be expected to have the effect of prohibiting or impairing any business practice of Acquiror or any acquisition of property by Acquiror or the conduct of business by Acquiror as currently conducted or as contemplated to be conducted as of the Closing other than such effects, individually or in the aggregate, which have not had an Acquiror Material Adverse Effect on the ability of Acquiror or Merger Sub to enter into, perform its obligations under this Agreement and consummate the Transactions.
(b) Other than Merger Sub, Acquiror does not own or have a right to acquire, directly or indirectly, any interest or investment (whether equity or debt) in any corporation, partnership, joint venture, business, trust or other entity.
(c) Except for (i) this Agreement and the agreements expressly contemplated hereby, (ii) as set forth on Schedule 5.09(c) and (iii) with respect to fees and expenses of Acquiror’s legal, financial and other advisors, Acquiror is not party to any Contract with any other Person that would require payments by Acquiror in excess of $100,000 in the aggregate with respect to any individual Contract or when taken together with all other Contracts (other than this Agreement and the agreements expressly contemplated hereby and Contracts set forth on Schedule 5.09(c)).
(d) There is no liability, debt or obligation against Acquiror or Merger Sub, except for (i) liabilities and obligations reflected or reserved for on Acquiror’s consolidated balance sheet as of June 30, 2025, or disclosed in the notes thereto (other than any such liabilities not reflected, reserved or disclosed as are not and would not be, in the aggregate, material to Acquiror and Merger Sub, taken as a whole), (ii) that have arisen since the date of Acquiror’s consolidated balance sheet as of June 30, 2025, in the ordinary course of the operation of business of the Acquiror and Merger Sub (other than any such liabilities as are or would be, in the aggregate, material to Acquiror and Merger Sub, taken as a whole) or (iii) disclosed in Schedule 5.09(d).
(e) Since its organization, Merger Sub has not conducted any business activities other than activities directed toward the accomplishment of the Merger. Except as set forth in Merger Sub’s organizational documents, there is no agreement, commitment, or Governmental Order binding upon Merger Sub or to which Merger Sub is a party which has had or would reasonably be expected to have the effect of prohibiting or impairing any business practice of Merger Sub or any acquisition of property by Merger Sub or the conduct of business by Merger Sub as currently conducted or as contemplated to be conducted as of the Closing other than such effects which have not had and would not reasonably be expected to have an Acquiror Material Adverse Effect.
Annex A-28
(f) Merger Sub does not own or have a right to acquire, directly or indirectly, any interest or investment (whether equity or debt) in any corporation, partnership, joint venture, business, trust or other entity.
(g) Merger Sub was formed solely for the purpose of effecting the Merger and has not engaged in any business activities or conducted any operations other than in connection with the Merger and has no, and at all times prior to the Effective Time except as contemplated by this Agreement or the Acquiror Support Agreement, will have no, assets, liabilities or obligations of any kind or nature whatsoever other than those incident to its formation.
(h) (i) Since the date of Acquiror’s incorporation, there has not been any change, development, condition, occurrence, event or effect relating to the Acquiror or Merger Sub that, individually or in the aggregate, resulted in, or would reasonably be expected to result in, an Acquiror Material Adverse Effect and (ii) from December 31, 2024, through the date of this Agreement, Acquiror and Merger Sub have not taken any action that would require the consent of the Company if such action had been taken after the date hereof.
Section 5.10 Information Supplied; Information Statement. None of the information supplied or to be supplied by the Acquiror or Merger Sub for inclusion in the Information Statement (together with any amendments or supplements thereto) will contain any untrue statement of a material fact or omit to state a material fact necessary to make the statements therein, in the light of the circumstances under which they were made, not misleading at the time such information is filed, submitted or made publicly available with the SEC; provided, however, that Acquiror makes no representations or warranties as to the information contained in or omitted from the Information Statement in reliance upon and in conformity with information furnished in writing to the Acquiror by or on behalf of the Company specifically for inclusion in the Information Statement.
Section 5.11 Litigation. As of the date of this Agreement, there are no material Actions pending or, to the Knowledge of the Acquiror, threatened against the Acquiror or, to the Knowledge of the Acquiror, any director, officer or employee of the Acquiror (in their capacity as such) and since the Acquiror’s date of incorporation there have not been any such material Actions. There are no material Actions pending or threatened by Acquiror against any other Person.
Section 5.12 No Outside Reliance. Notwithstanding anything contained in this Article V or any other provision hereof, Acquiror and its Affiliates acknowledge and agree that Acquiror has made its own investigation of the Company and that neither the Company nor any of its Affiliates or any of their respective directors, officers, employees, stockholders, partners, members, agents or Representatives is making any representation or warranty whatsoever, express or implied, beyond those expressly given by the Company in Article IV, including any implied warranty or representation as to condition, merchantability, suitability or fitness for a particular purpose or trade as to any of the assets of the Company. Without limiting the generality of the foregoing, it is understood that any cost or other estimates, financial or other projections or other predictions that may be contained or referred to in the Schedules or elsewhere, as well as any information, documents or other materials (including any such materials contained in any “data room” (whether or not accessed by Acquiror or its Representatives)) or management presentations that have been or shall hereafter be provided to Acquiror or any of its Affiliates, agents or Representatives are not and will not be deemed to be representations or warranties of the Company, and no representation or warranty is made as to the accuracy or completeness of any of the foregoing except as may be expressly set forth in Article IV of this Agreement. Except as otherwise expressly set forth in this Agreement, Acquiror understands and agrees that any assets, properties and business of the Company are furnished “as is,” “where is” and subject to and except as otherwise provided in the representations and warranties contained in Article IV, with all faults and without any other representation or warranty of any nature whatsoever.
Section 5.13 Capitalization.
(a) As of the date hereof, the authorized capital stock of Acquiror consists of (i) 100,000,000 shares of Acquiror Common Stock, of which (A) 12,575,983 shares of Acquiror Common Stock are issued and outstanding as of the date of this Agreement and (ii) 10,000,000 preferred shares of Acquiror, par value $0.01, none of which are issued and outstanding as of the date of this Agreement. All of the issued and outstanding shares of Acquiror Common Stock (1) have been duly authorized and validly issued and are fully paid and nonassessable, (2) were issued in compliance in all material respects with applicable Law, (3) were not issued in breach or violation of any preemptive rights or Contract and (4) are fully vested and not otherwise subject to a substantial risk of forfeiture within the meaning of Code Section 83.
Annex A-29
(b) Except for this Agreement and except as disclosed in Schedule 5.13(b)(i), there are (i) no subscriptions, calls, options, warrants, rights or other securities convertible into or exchangeable or exercisable for Acquiror Common Stock or the equity interests of Acquiror, or any other Contracts to which Acquiror is a party or by which Acquiror is bound obligating Acquiror to issue or sell any shares of capital stock of, other equity interests in or debt securities of, Acquiror, and (ii) no equity equivalents, stock appreciation rights, phantom stock ownership interests or similar rights in Acquiror. Except as disclosed in Schedule 5.13(b)(ii) or the Acquiror Organizational Documents, there are no outstanding contractual obligations of Acquiror to repurchase, redeem or otherwise acquire any securities or equity interests of Acquiror. There are no outstanding bonds, debentures, notes or other indebtedness of Acquiror having the right to vote (or convertible into, or exchangeable for, securities having the right to vote) on any matter for which Acquiror Stockholders may vote. Except as disclosed in Schedule 5.13(b)(iii), Acquiror is not a party to any stockholders agreement, voting agreement or registration rights agreement relating to Acquiror Common Stock or any other equity interests of Acquiror. Other than Merger Sub, Acquiror does not own any capital stock or any other equity interests in any other Person or has any right, option, warrant, conversion right, stock appreciation right, redemption right, repurchase right, agreement, arrangement or commitment of any character under which a Person is or may become obligated to issue or sell, or give any right to subscribe for or acquire, or in any way dispose of, any shares of the capital stock or other equity interests, or any securities or obligations exercisable or exchangeable for or convertible into any shares of the capital stock or other equity interests, of such Person. There are no securities or instruments issued by or to which the Acquiror is a party containing anti-dilution or similar provisions that will be triggered by the consummation of the transactions contemplated by this Agreement that have not been or will not be waived on or prior to the Closing Date.
(c) All of the issued and outstanding Equity Securities of Merger Sub and Bio Sub are held by Acquiror as of the date of this Agreement. All outstanding Equity Securities of such Merger Sub and Bio Sub are validly issued, fully paid and non-assessable, and are not subject to preemptive rights or any other Liens (other than Liens arising pursuant to applicable Securities Laws).
Section 5.14 NYSE American Quotation. The issued and outstanding shares of Acquiror Common Stock are registered pursuant to Section 12(b) of the Exchange Act and are listed for trading on NYSE American under the symbol “APUS.” Acquiror is in compliance in all material respects with the rules of the NYSE American and there is no action or proceeding pending or, to the Knowledge of Acquiror, threatened against Acquiror by NYSE American, the Financial Industry Regulatory Authority or the SEC with respect to any intention by such entity to deregister the Acquiror Common Stock or terminate the listing of Acquiror Common Stock on NYSE American. None of Acquiror or its Affiliates has taken any action in an attempt to terminate the registration of the Acquiror Common Stock under the Exchange Act except as contemplated by this Agreement.
Section 5.15 Affiliate Agreements. Except as disclosed in the Acquiror SEC Reports, neither of the Acquiror nor Merger Sub is a party to any transaction, agreement, arrangement or understanding with any (a) present or former executive officer or director of either of the Acquiror or Merger Sub, (b) beneficial owner (within the meaning of Section 13(d) of the Exchange Act) of five (5%) percent or more of the capital stock or equity interests of Acquiror or (c) Affiliate, “associate” or member of the “immediate family” (as such terms are respectively defined in Rules 12b-2 and 16a-1 of the Exchange Act) of any of the foregoing.
Section 5.16 Anti-Bribery; Economic Sanctions.
(a) Since their respective dates of incorporation, Acquiror and Merger Sub have complied with all applicable Anti-Bribery Laws. Since their respective dates of incorporation, neither Acquiror nor Merger Sub, nor to the Knowledge of the Acquiror, any of their respective Representatives, have directly or indirectly paid, offered or promised to pay, or authorized or ratified the payment, directly or indirectly, of any monies or anything of value to any Public Official for the purpose of influencing any act or decision of such official or of any Governmental Authority to obtain or retain business, or direct business to any person or to secure any other improper benefit or advantage.
(b) Neither Acquiror nor Merger Sub are Sanctioned Persons or located, organized, or ordinarily reside in an Embargoed Jurisdiction.
(c) Acquiror and Merger Sub maintain in effect written policies, procedures and internal controls, including an internal controls system, that are reasonably designed to promote compliance with applicable International Trade Laws and Anti-Bribery Laws.
Annex A-30
Section 5.17 Labor and Employment.
(a) Schedule 5.17(a) sets forth a true, correct and complete list of all current employees of the Acquiror as of a date not more than five (5) days before the Closing Date, including any employee who is on a leave of absence of any nature, authorized or unauthorized, and sets forth for each such individual the following: (i) name; (ii) title or position (including whether full or part time); (iii) hire date; (iv) current annual base compensation rate; (v) commission, bonus or other incentive based compensation; and (vi) the term of their employment (indefinite or definite). As of the date hereof, all compensation, including wages, commissions and bonuses, due and payable to all employees of the Acquiror for services performed on or prior to the date hereof, has been paid in full. As of the date hereof, Merger Sub has not had and does not have any employees or independent contractors.
(b) Except as set forth on Schedule 5.17(b), (i) there are no material Actions pending or, to the knowledge of the Acquiror, threatened in writing against the Acquiror alleging violations of any Law pertaining to labor relations or employment matters, by any of their respective current or former employees, which Actions would be material to the Acquiror, taken as a whole; (ii) the Acquiror is not, nor has the Acquiror been for the past two (2) years, a party to, bound by, or negotiating any collective bargaining agreement or other contract with a union, works council or labor organization applicable to persons employed by the Acquiror, nor, to the knowledge of the Acquiror, is there a union organizing campaign in progress with respect to any such employees; (iii) there are no unfair labor practice complaints pending against the Acquiror before any Governmental Authority; and (iv) for the past two (2) years there has not been, nor, to the knowledge of the Acquiror, has there been threatened in writing, any strike, slowdown, work stoppage, lockout, concerted refusal to work overtime or other material labor dispute affecting any employees of the Acquiror.
(c) The Acquiror is, and for the last two (2) years has been, in compliance in all material respects with all applicable Laws relating to the employment, employment practices, employment discrimination, terms and conditions of employment, mass layoffs and plant closings (including the Worker Adjustment and Retraining Notification Act of 1988, as amended, or any similar Laws), immigration, meal and rest breaks, pay equity, workers’ compensation, family and medical leave, and occupational safety and health requirements, including those related to wages, hours, collective bargaining and the payment and withholding of Taxes and other sums as required by the appropriate Governmental Authority and are not liable for any arrears of wages, Taxes, penalties or other sums for failure to comply with any of the foregoing.
(d) To the Knowledge of the Acquiror, no current or former employee or independent contractor of the Acquiror is in any material respect in violation of any term of any employment agreement, nondisclosure agreement, common law nondisclosure obligation, fiduciary duty, noncompetition agreement, nonsolicitation agreement, restrictive covenant or other obligation: (i) owed to the Acquiror; or (ii) owed to any third party with respect to such Person’s right to be employed or engaged by the Acquiror. To the Knowledge of the Acquiror, no current employee of the Acquiror with annualized base compensation at or above $250,000, has given notice to the Acquiror that the employee intends to terminate his or her employment prior to the one year anniversary of the Closing.
(e) The Acquiror has promptly, thoroughly and impartially investigated all sexual harassment, or other unlawful discrimination or unlawful retaliation, complaints made by or against employees of the Acquiror, in each case in connection with their employment with the Acquiror, of which it has been made aware in the past two (2) years. With respect to each such complaint to the extent warranted based on the Acquiror’s investigation, the Acquiror has taken prompt corrective action that is reasonably calculated to prevent further improper action. The Acquiror does not reasonably expect any material liabilities with respect to any such complaints and, to the Knowledge of the Acquiror, there are no such complaints relating to officers, directors, employees, contractors, or agents of the Acquiror relating to their employment with or service to the Acquiror, that, if known to the public, would bring the Acquiror into material disrepute.
(f) Except as would not result in material liability for the Acquiror, in the past two (2) years the Acquiror has fully and timely paid all (i) wages, salaries, wage premiums, commissions, overtime, bonuses, severance and termination payments, fees, and other compensation that has come due and payable to its current or former employees and independent contractors under applicable Laws, Contract or Acquiror policy, and (ii) fines, Taxes, interest, or other penalties for any failure to pay or delinquency in paying such compensation.
Annex A-31
Section 5.18 No Other Representations or Warranties. The representations and warranties made by Acquiror and Merger Sub in this Article V are the exclusive representations and warranties made by Acquiror, Merger Sub, their Affiliates, and their respective Representatives. Except for the representations and warranties contained in this Article V, neither Acquiror nor Merger Sub, nor any other Person, has made or makes any other express or implied representation or warranty, either written or oral, on behalf of Acquiror or Merger Sub, to the accuracy or completeness of any information regarding Acquiror or Merger Sub available to the other parties or their respective Representatives and expressly disclaims any such other representations or warranties. Without limiting the foregoing, neither Acquiror nor Merger Sub, nor any other Person, makes or has made any representation or warranty to the other parties hereto with respect to, and shall have no liability in respect of, (a) any financial projection, forecast, estimate, budget or prospect information relating to Acquiror or Merger Sub or (b) any oral or, except for the representations and warranties expressly made by Acquiror or Merger Sub in this Article V, written information made available to the other parties hereto in the course of their evaluation of Acquiror and Merger Sub and the negotiation of this Agreement or in the course of the Transactions. Each of Acquiror and Merger Sub hereby acknowledge and agree with the statements and provisions set forth in Section 4.26.
Article VI
COVENANTS OF THE COMPANY
Section 6.01 Financial Statements; Stockholder Approval; Other Actions.
(a) The Company agrees to use reasonable best efforts to provide Acquiror, as promptly as reasonably practicable after the date hereof, and in any event by no later than seventy-five (75) days from the Closing, unaudited interim financial statements, including consolidated balance sheets, statements of operations, statements of cash flows, and statements of stockholders’ equity of the Company on a consolidated basis as of and for the nine (9) months ended 2025, prepared in accordance with GAAP and Regulation S-X, and any other financial statements (other than financial statements of Acquiror or Merger Sub) required to be included in the Information Statement in accordance with the rules and regulations of the SEC, including pro forma financial statements. The Company shall be available to, and the Company shall use reasonable best efforts to make their officers and employees available to, in each case, during normal business hours and upon reasonable advanced notice, Acquiror and its counsel in connection with (i) the drafting of the Information Statement and (ii) responding in a timely manner to comments on the Information Statement from the SEC. Without limiting the generality of the foregoing, the Company shall reasonably cooperate with Acquiror in connection with Acquiror’s preparation for inclusion in the Information Statement of pro forma financial statements that comply with the requirements of Regulation S-X under the rules and regulations of the SEC (as interpreted by the staff of the SEC) to the extent such pro forma financial statements are required by Rule 14c-3 under the Exchange Act.
(b) Until the expiration of the Waiting Period, the Company will give Acquiror prompt written notice of any action taken or not taken by the Company or of any development regarding the Company, in any such case which is known by the Company, that would cause the Information Statement to contain an untrue statement of a material fact or omit to state a material fact necessary in order to make the statements, in light of the circumstances under which they were made, not misleading; provided, that, if any such action shall be taken or fail to be taken or such development shall otherwise occur, Acquiror and the Company shall cooperate fully to cause an amendment or supplement to be made promptly to the Information Statement, such that the Information Statement no longer contains an untrue statement of a material fact or omit to state a material fact necessary in order to make the statements, in light of the circumstances under which they were made, not misleading; provided, further, however, that no information received by Acquiror pursuant to this Section 6.01(b) shall operate as a waiver or otherwise affect any representation, warranty or agreement given or made by the party who disclosed such information, and no such information shall be deemed to change, supplement or amend the Schedules.
(c) Prior to the execution of this Agreement, the Company shall have obtained the requisite approval of the Company Board and its stockholders, by written consent (the “Company Stockholder Approval”), (i) approving the entry by the Company into this Agreement and the consummation of the Transactions, (ii) intentionally omitted, and (iii) approving and authorizing the designation of the individual to serve as the Acquiror’s chief executive officer, in accordance with Section 2.05(c).
Annex A-32
Section 6.02 Asset Covenant. The Company hereby covenants and agrees that, as of the date of this Agreement (which is also the Closing Date), the representations and warranties set forth in Section 4.26 are true, correct, and complete in all respects, and are hereby reaffirmed by the Company as of such date.
Article VII
COVENANTS OF ACQUIROR
Section 7.01 Access and Information. From and after the Closing until the Conversion Effective Time (the “Interim Period”), Acquiror will give, and will cause its Representatives to give, the Company, at reasonable times during normal business hours and upon reasonable intervals and notice, reasonable access to all offices and other facilities and to appropriate employees, properties, Contracts, agreements, commitments, books and records, financial and operating data and other information (including Tax Returns, internal working papers, client files, client Contracts and director service agreements), of or pertaining to Acquiror, as the Company or its Representatives may reasonably request regarding Acquiror, and its respective business, assets, liabilities, financial condition, prospects, operations, management, employees and other aspects (including unaudited quarterly financial statements, including a consolidated quarterly balance sheet and income statement, a copy of each material report, schedule and other document filed with or received by a Governmental Authority under the requirements of applicable securities Laws, and independent public accountants’ work papers (subject to the consent or any other conditions required by such accountants, if any)) and cause each of Acquiror’s Representatives to reasonably cooperate with the Company and its Representatives in their investigation; provided, however, that the Company and its Representatives will conduct any such activities in such a manner as not to unreasonably interfere with the business or operations of Acquiror and Acquiror will not be required to provide information it reasonably determines that it cannot provide as a matter of Law, Contract, or protection of attorney-client or similar privilege. No information or knowledge obtained by the Company in any investigation conducted under the access contemplated by this Section 7.01 will affect or be deemed to modify any representation or warranty of Acquiror set forth in this Agreement or otherwise impair the rights and remedies available to the Company.
Section 7.02 Indemnification and Insurance.
(a) From and after the Effective Time, Acquiror and the Surviving Corporation shall indemnify and hold harmless each present and former director or officer of the Company, and each present director or officer of Acquiror, against any costs or expenses (including reasonable attorneys’ fees), judgments, fines, losses, claims, damages or liabilities incurred in connection with any Action, whether civil, criminal, administrative or investigative, arising out of or pertaining to matters existing or occurring at or prior to the Effective Time, whether asserted or claimed prior to, at or after the Effective Time, to the fullest extent that the Company or Acquiror, as applicable, would have been permitted under applicable Law, the Company Organizational Documents or Acquiror Organizational Documents and indemnification agreements (or Contracts containing similar indemnification provisions) in effect on the date of this Agreement to indemnify such Person (and advance expenses as incurred in defense of any Action to the fullest extent permitted under applicable Law). Without limiting the foregoing, Acquiror shall, and shall cause the Surviving Corporation to, for a period of not less than six years from the Effective Time, (i) maintain provisions in its certificate of incorporation, bylaws, other organization documents and indemnification agreements (or Contracts containing similar indemnification provisions), to the extent applicable, concerning the indemnification and exculpation (and provisions relating to expense advancement) of officers and directors that are no less favorable to those Persons than the provisions of the Acquiror Organizational Documents, and such indemnification agreements (or Contracts containing similar indemnification provisions), to the extent applicable, as of the date of this Agreement and (ii) not amend, repeal, terminate or otherwise modify such provisions in any respect that would adversely affect the rights of those Persons thereunder, in each case, except as required by Law. Acquiror shall assume, and be liable for, and shall cause the Surviving Corporation and its Subsidiaries to honor, each of the covenants in this Section 7.02.
(b) For a period of six years from the Effective Time, Acquiror shall, or shall cause the Surviving Corporation to, maintain in effect directors’ and officers’ liability insurance covering those Persons who are currently covered by the Company’s and Acquiror’s directors’ and officers’ liability insurance policies (true, correct and complete copies of which have been heretofore made available to Acquiror or its agents or Representatives) on terms not less favorable than the terms of such current insurance coverage; provided, however, that (i) Acquiror may cause coverage to be extended under the Company’s and/or Acquiror’s current directors’ and officers’ liability insurance by obtaining a six-year “tail” policy containing terms not materially less favorable than the terms of such current insurance coverage with respect to claims existing or occurring at or prior to the Effective Time, provided that (A) in no event shall
Annex A-33
Acquiror be required to expend on the premium thereof in excess of 350% of the aggregate annual premiums currently payable by the Company or Acquiror, as applicable, with respect to such current policy (the “Premium Cap”), and (B) if such minimum coverage under any such “tail” policy is or becomes not available at the Premium Cap, then any such tail policy shall contain the maximum coverage available at the Premium Cap; and (ii) if any claim is asserted or made within such six-year period, any insurance required to be maintained under this Section 7.02 shall be continued in respect of such claim until the final disposition thereof.
(c) This Section 7.02 shall survive the consummation of the Merger indefinitely and shall be binding, jointly and severally, on Acquiror and the Surviving Corporation and all successors and assigns of Acquiror and the Surviving Corporation. In the event that Acquiror, the Surviving Corporation or any of their respective successors or assigns consolidates with or merges into any other Person and shall not be the continuing or surviving corporation or entity of such consolidation or merger or transfers or conveys all or substantially all of its properties and assets to any Person, then, and in each such case, Acquiror and the Surviving Corporation shall ensure that proper provision shall be made so that the successors and assigns of Acquiror or the Surviving Corporation, as the case may be, shall succeed to the obligations set forth in this Section 7.02. The obligations of Acquiror and the Surviving Corporation under this Section 7.02 shall not be terminated or modified in such a manner as to materially and adversely affect any present and former director and officer of the Company or any present director or officer of Acquiror without the consent of the affected Person (it being expressly agreed that the covered directors and officers of the Company and the covered directors and officers of the Acquiror shall be third party beneficiaries of this Section 7.02).
Section 7.03 Additional Insurance Matters. The Acquiror shall maintain directors’ and officers’ liability insurance (such insurance to be reasonably acceptable to the Company) that shall be effective as of Closing and will cover those Persons who will be the directors and officers of Acquiror and its Subsidiaries (including the officers of the Company) at and after the Closing on terms customary for a typical directors’ and officers’ liability insurance policy for a company whose equity is listed on NYSE American which policy has a scope and amount of coverage that is reasonably appropriate for a company of similar characteristics (including the line of business and revenues) as Acquiror and its Subsidiaries (including the Company).
Section 7.04 Conduct of Business.
(a) Unless the Company will otherwise consent in writing (such consent not to be unreasonably withheld, conditioned or delayed), during the Interim Period, except as expressly contemplated by this Agreement, Acquiror and Bio Sub will (i) conduct their respective businesses, in all material respects, in the ordinary course of business consistent with past practice, (ii) comply with all Laws applicable to Acquiror and their respective businesses, assets and employees, and (iii) take all commercially reasonable measures necessary or appropriate to preserve intact, in all material respects, their respective business organizations, to keep available the services of the key employees, and to preserve the possession, control and condition of their respective material assets, all as consistent with past practice.
(b) Without limiting the generality of Section 7.04(a) and except as contemplated by the terms of this Agreement, during the Interim Period, without the prior written consent of the Company (such consent not to be unreasonably withheld, conditioned or delayed), Acquiror will not:
(i) amend, waive or otherwise change, in any respect, the Acquiror Organizational Documents;
(ii) authorize for issuance, issue, grant, sell, pledge, dispose of or propose to issue, grant, sell, pledge or dispose of any of its equity securities or any options, warrants, commitments, subscriptions or rights of any kind to acquire or sell any of its equity securities, or other securities, including any securities convertible into or exchangeable for any of its shares or other equity securities or securities of any class and any other equity-based awards, or engage in any hedging transaction with a third Person with respect to such securities;
(iii) split, combine, recapitalize or reclassify any of its shares or other equity interests or issue any other securities or pay or set aside any dividend or other distribution (whether in cash, equity or property or any combination) in respect of its shares or other equity interests, or directly or indirectly redeem, purchase or otherwise acquire or offer to acquire any of its securities;
Annex A-34
(iv) incur, create, assume, prepay or otherwise become liable for any Indebtedness (directly, contingently or otherwise) in excess of $100,000 individually or $250,000 in the aggregate, make a loan or advance to or investment in any third party, or guarantee or endorse any Indebtedness, Liability or obligation of any Person in excess of $100,000 individually or $250,000 in the aggregate;
(v) (A) materially increase the wages, salaries or compensation of its employees, in any event not in the aggregate by more than five percent (5%), (B) make or commit to make any bonus payment (whether in cash, property or securities) to any employee, or (C) enter into, establish, materially amend or terminate any Acquiror Benefit Plan with, for or in respect of any current consultant, officer, manager director or employee, in each case of (A) – (C) other than as required by applicable Law, under the terms of any Acquiror Benefit Plans or in the ordinary course of business consistent with past practice;
(vi) make, change, or rescind any material election relating to Taxes, settle any claim, action, suit, litigation, proceeding, arbitration, investigation, audit or controversy relating to Taxes, file any amended Tax Return or claim for refund, or make any change in its accounting or Tax policies or procedures, in each case except as required by applicable Law or in compliance with GAAP;
(vii) transfer or license to any Person or otherwise extend, materially amend or modify, permit to lapse or fail to preserve any material Acquiror Intellectual Property, or disclose to any Person who has not entered into a confidentiality agreement any trade secrets;
(viii) terminate or waive or assign any right under, any Acquiror material contract or enter into any Contract that would be a material contract;
(ix) fail to maintain its books, accounts and records in all material respects in the ordinary course of business consistent with past practice;
(x) establish any Subsidiary or enter into any new line of business;
(xi) fail to use commercially reasonable efforts to keep in force insurance policies or replacement or revised policies providing insurance coverage with respect to its assets, operations and activities in such amount and scope of coverage substantially similar to that which is currently in effect;
(xii) change its fiscal year, revalue any of its material assets or make any change in accounting methods, principles or practices, except to the extent required to comply with GAAP and after consulting with Acquiror’s outside auditors;
(xiii) waive, release, assign, settle or compromise any claim, action or proceeding (including any suit, Action, claim, proceeding or investigation relating to this Agreement or the Merger and the other contemplated transactions);
(xiv) close or materially reduce its activities, or effect any layoff or other personnel reduction or change, at any of its facilities;
(xv) acquire, including by merger, consolidation, acquisition of equity interests or assets, or any other form of business combination, any corporation, partnership, limited liability company, other business organization or any division, or any material amount of assets outside the ordinary course of business consistent with past practice;
(xvi) make capital expenditures in excess of $50,000 (individually for any project (or set of related projects) or $100,000 in the aggregate);
(xvii) authorize, recommend, propose, or announce an intention to adopt a plan of complete or partial liquidation, dissolution, merger, consolidation, restructuring, recapitalization or other reorganization (other than with respect to the Merger);
(xviii) voluntarily incur any Liability or obligation (whether absolute, accrued, contingent or otherwise) in excess of $50,000 individually or $100,000 in the aggregate other than under the terms of a Acquiror material contract or Acquiror Benefit Plan;
Annex A-35
(xix) sell, lease, license, transfer, exchange or swap, mortgage or otherwise pledge or encumber (including securitizations), or otherwise dispose of any portion of its properties, assets or rights;
(xx) enter into any agreement, understanding or arrangement with respect to the voting of Equity Securities of Acquiror;
(xxi) take any action that would reasonably be expected to significantly delay or impair the obtaining of any consents of any Governmental Authority to be obtained in connection with this Agreement;
(xxii) hire any employee, officer, consultant, freelancer, independent contractor or sub-contractor, or adopt or enter into any new employee benefit or compensatory plan, policy, program, agreement, trust or arrangement;
(xxiii) other than in the ordinary course of business (A) pay or promise to pay, fund any new, enter into or make any grant of any severance, change in control, retention or termination payment to any director, officer, employee, consultant, freelancer, independent contractor or sub-contractor of Acquiror, (B) take any Action to accelerate any material payments or benefits, or the funding of any material payments or benefits, payable or to become payable to any director, officer, other employee of Acquiror, or (C) take any action to materially increase any compensation or material benefits of any director, officer, other employee, consultant, freelancer, independent contractor or sub-contractor of Acquiror;
(xxiv) accelerate the collection of any trade receivables or delay the payment of trade payables or any other liabilities other than in the ordinary course of business consistent with past practice;
(xxv) enter into, amend, waive or terminate (other than terminations in accordance with their terms) any transaction with any employee, contractor or director (other than compensation and benefits and advancement of expenses, in each case, provided in the ordinary course of business consistent with past practice);
(xxvi) maintain the existing relations and goodwill of Acquiror with customers, suppliers, distributors and creditors of Acquiror and use commercially reasonable efforts to maintain all insurance policies of Acquiror or equivalent substitutes;
(xxvii) use any portion of the proceeds received from the Private Placement Offering and any and all other financings consummated by Acquiror or the Surviving Corporation other than such amount allocated to the Bio Sub in accordance with the Side Letter;
(xxviii) directly or indirectly engage in any capital raising transaction; or
(xxix) agree or commit to do, or resolve, authorize or approve any action to do any of the foregoing, or take any action or omission that would result in any of the foregoing.
Section 7.05 Director and Officer Appointments. Except as otherwise agreed in writing by the Company and Acquiror prior to the Closing, and conditioned upon the occurrence of the Closing, subject to any limitation imposed under applicable Laws and NYSE American listing requirements, Acquiror shall take all actions necessary or appropriate to cause (a) the number of directors constituting the Acquiror Board to be seven (7), (b) the individuals set forth on Schedule 7.05 to be elected as members of the Acquiror Board, effective as of the Closing and (c) the individuals set forth on Schedule 7.05 to be the executive officers of Acquiror effective as of the Closing. On the Closing Date, Acquiror shall enter into customary indemnification agreements reasonably satisfactory to the Company with the individuals set forth on Schedule 7.05, which indemnification agreements shall continue to be effective following the Closing.
Section 7.06 Written Consent. In accordance with applicable Laws, including Section 228 of the DGCL, and the Acquiror Organizational Documents, immediately after the execution of this Agreement, in lieu of calling a meeting of the Acquiror Stockholders, the Acquiror shall obtain the Acquiror Stockholder Approval from the Majority Holders, as set forth in the Acquiror Support Agreement, and the Acquiror shall promptly provide the Company with a copy of such Written Consent. In connection with the Written Consent, the Acquiror shall take all actions necessary or advisable to comply, and shall comply in all respects, with the DGCL, including Section 228 thereof, and the Acquiror Organizational Documents. The Written Consent shall constitute the required Acquiror Stockholder Approvals of the Proposals; provided, however, that none of the Proposals approved by the Written Consent shall be implemented prior to the expiration of the 20 calendar day waiting period required by Rule 14c-2 promulgated under
Annex A-36
the Exchange Act (the “Waiting Period”) following the mailing of the Information Statement in definitive form (the “Definitive Information Statement”) to the Acquiror Stockholders as contemplated by Rule 14c-5 under the Exchange Act. Following the expiration of the Waiting Period, the Proposals approved by the Written Consent, including the Conversion, shall become the approved and binding corporate actions of the Acquiror (the “Action Effective Time”).
Section 7.07 Post-Closing Governance of the Surviving Corporations. The members of the Company Board immediately prior to the Effective Time shall continue to serve in their respective capacities with respect to the Surviving Corporation following the Closing. During the Interim Period, the Company Board shall have sole and complete authority and control over the business and affairs of the Surviving Corporation, including but not limited to (a) to manage and direct all operations of the Surviving Corporation, (b) control and access to all bank accounts and other financial accounts of the Surviving Corporation, (c) the authority to approve or disapprove all expenditures, obligations, and other commitments of funds, (d) authorize any hiring, termination, or compensation decisions with respect to personnel, and (e) approve the receipt, use, and disbursement of any and all funds or other assets of the Surviving Corporation.
Section 7.08 Bio Sub and Financing Allocations.
(a) Promptly following the Closing, and in any event no less than five (5) Business Days following the Closing, Acquiror shall cause the assets and liabilities of Acquiror existing immediately prior to the Closing to be transferred to Bio Sub, the purpose of which shall be to continue the current business and operations of Acquiror, which is a clinical-stage biopharmaceutical company focused on the development of Apitox, an intradermally administered bee venom-based toxin (the “Bio Business”). All Intellectual Property, know-how, and proprietary information used in Acquiror’s business as of the Closing Date shall be transferred to Bio Sub and shall not be licensed, assigned, or otherwise disposed of without the prior written consent of the Bio Business Representative. The Chief Executive Officer and President of the Bio Sub shall be Erik Emerson.
(b) Until the earlier of the Funding Cap being reached or six (6) months following the Closing (the “Post-Closing Period”), the Acquiror shall cause the Bio Business to be preserved and operated in a manner consistent in all material respects with the past practices of the Acquiror prior to the Closing. The Bio Business shall be maintained within the Bio Sub and shall continue to pursue the clinical development of Apitox, an intradermally administered bee venom-based toxin, and related biopharmaceutical initiatives. During the Post-Closing Period, except as otherwise expressly consented to by the Bio Business Representative, the Acquiror shall not, and the Acquiror shall not cause the Bio Sub to:
(i) Amend or propose to amend Bio Sub’s certificate of incorporation, bylaws, or similar organizational documents;
(ii) split, combine, reclassify, or otherwise alter the Bio Sub’s outstanding capital stock, equity interests, voting securities, or issue or authorize any securities in substitution thereof;
(iii) issue, sell, pledge, dispose of, or encumber any shares of the Bio Sub’s capital stock, securities convertible into such stock, or any options, warrants, calls, or rights to acquire shares;
(iv) sell, lease, license, transfer, mortgage, pledge, or otherwise dispose of or encumber any material assets or properties of the Bio Sub;
(v) discontinue any material business lines of the Bio Business;
(vi) enter into, amend, modify, terminate, or waive rights under any material contract, lease, or agreement;
(vii) dispose of, fail to maintain, or grant licenses to any intellectual property rights, or subject any assets to new liens or encumbrances;
(viii) engage in or become a party to any merger, consolidation, share exchange, business combination, recapitalization, or similar transaction involving the Bio Sub;
(ix) acquire by merger, consolidation, or purchase any business, entity or substantial assets thereof;
Annex A-37
(x) enter into any collective bargaining agreement, labor union contract, or similar;
(xi) commence, settle, or compromise any material litigation, claims, or legal proceedings, or waive/release material rights;
(xii) terminate the employment of any employee of Acquiror, as of the Closing Date (except for cause (as defined in the applicable employment agreement or Bio Sub’s policies));
(xiii) reduce in force, layoff or make any material changes to any compensation or benefits plans;
(xiv) make, change, or revoke any tax election;
(xv) settle or compromise any tax liability;
(xvi) extend or waive any limitations periods for tax assessments; or
(xvii) fail to maintain in full force all material insurance policies covering the Bio Sub.
(c) The board of directors of Bio Sub shall, at all times until the end of the Post-Closing Period, consist solely of the individuals serving as directors of Acquiror immediately prior to the Closing, unless otherwise agreed in writing by the Bio Business Representative.
(d) The Acquiror shall use its best efforts to consummate a private placement offering in an aggregate amount of up to $120,900,000.00 (the “Private Placement Offering”).
(e) Except as set forth in the Amended and Restated Side Letter Agreement between the Acquiror, Bio Sub and the Company dated December 1, 2025 (the “Side Letter”), all proceeds received from the Private Placement Offering shall be deposited into an account designated by the Company.
(f) Reserved.
(g) If the Acquiror has not consummated the Private Placement Offering prior to or contemporaneously with the Closing, Bio Sub shall deposit $2,500,000 from its available cash into an account designated by the Company (the “Bio Sub Loan”) within 2 Business Days, to be used for general operational purposes of TechyTrade. Such Bio Sub Loan shall be repaid from the proceeds of any subsequent financing received by Acquiror, and such repayment shall be made in addition to, and not in lieu of, any amounts allocated to the Bio Business pursuant to the Side Letter. The remaining available cash of Bio Sub shall be retained by Bio Sub and used solely to advance the Bio Business.
(h) The parties have appointed Erik Emerson as the Bio Business Representative, who shall have the right to monitor the Acquiror’s compliance with its obligations in this Section 7.08. Following the Closing, the Bio Business Representative shall be entitled to receive regular updates from the Acquiror’s management and Bio Sub’s management, including quarterly reports on operations, financing allocations, and material developments. The covenants in this Section 7.08 shall be binding on Acquiror and its successors and shall survive the Closing. In the event of a breach of this Section 7.08, the Bio Business Representative shall have the right to seek specific performance, injunctive relief, or other equitable remedies, and shall have standing to bring legal action in any court of competent jurisdiction to enforce the obligations set forth herein. The parties expressly agree that the Bio Business Representative shall be a third-party beneficiary of this Section 7.08 with full enforcement rights.
(i) During the Post-Closing Period, the management of the Bio Sub shall cause the Bio Business to be operated in a manner consistent in all material respects with the past practices of the Acquiror prior to the Closing, and shall not incur Indebtedness, which individually or in the aggregate exceeds $2,000,000 without the prior written consent of the CEO of the Acquiror.
(j) Acquiror grants an irrevocable proxy to the Bio Sub Representative in connection with voting the shares of Bio Sub owned by Acquiror to be effective upon the termination of the Interim Period. In any sale, carve-out, split-off, spin-off transaction or other change in control transaction, Acquiror shall not issue or sell equity interests in the Bio Sub for less than the then applicable market value.
Annex A-38
Section 7.09 Reserved.
Section 7.10 YSE American Listing Application(a). Prior to the Conversion, Acquiror shall obtain conditional approval of its listing application from NYSE American in connection with the Transactions, including any required new listing application due to a change in control or Reverse Merger (as defined in Section 341 of the NYSE American Company Guide). Immediately prior to the Conversion, Acquiror shall satisfy all applicable continuing listing requirements of NYSE American (or be granted a grace period therefrom), shall not have received any notice of non-compliance, and the Acquiror Common Stock, including the Merger Consideration, shall have been approved for listing on NYSE American.
Section 7.11 Form S-8 Filing Related to 2024 Plan Share Increase. Within five (5) Business Days following the Action Effective Time, Acquiror shall file, or cause to be filed, with the SEC a registration statement on Form S-8 to register the additional shares of Acquiror Common Stock reserved for issuance under the 2024 Equity Incentive Plan pursuant to the 2024 Plan Share Increase.
Article VIII
joint COVENANTS
Section 8.01 Support of Transaction. Without limiting any covenant contained in Article VI or Article VII, including the obligations of the Company and Acquiror with respect to the notifications, filings, reaffirmations and applications described in Section 6.01, which obligations shall control to the extent of any conflict with the succeeding provisions of this Section 8.01, Acquiror and the Company shall each, and Acquiror shall cause Merger Sub to: (i) use commercially reasonable efforts to assemble, prepare and file any information (and, as needed, to supplement such information) as may be reasonably necessary to obtain as promptly as practicable all governmental and regulatory consents required to be obtained in connection with the Transactions, (ii) use commercially reasonable efforts to obtain all material consents and approvals of third parties that any of Acquiror, the Company, or their respective Affiliates are required to obtain in order to consummate the Transactions, including any required approvals of parties to Material Contracts with the Company as specified in Schedule 8.01, and (iii) take such other action as may reasonably be necessary or as another party may reasonably request to satisfy the conditions of Article IX or otherwise to comply with this Agreement and to consummate the Transactions as soon as practicable. Notwithstanding the foregoing, in no event shall Acquiror, Merger Sub or the Company be obligated to bear any expense or pay any fee or grant any concession in connection with obtaining any consents, authorizations or approvals pursuant to the terms of any Contract to which the Company is a party or otherwise in connection with the consummation of the Transactions.
Section 8.02 Preparation of the Information Statement.
(a) As promptly as reasonably practicable after the date hereof, the Acquiror shall prepare and file with the SEC a written information statement of the type contemplated by Rule 14c-2 of the Exchange Act containing the information specified in Schedule 14C under the Exchange Act concerning the Written Consent, the Proposals approved by the Majority Holders and the Merger, and the notice of action by written consent required by Section 228(e) of the DGCL (as amended or supplemented from time to time, the “Information Statement”).
(b) The Acquiror shall provide the Company with a draft of the Information Statement (and any amendment or supplement thereto) prior to filing with the SEC. The Company shall provide the Acquiror with all information concerning the Company and its affiliates as may be reasonably requested by the Acquiror and is customarily included in an information statement. The Company shall promptly correct any information with respect to it or provided by it for use in the Information Statement if and to the extent, in the absence of such a correction, the Information Statement would contain a misstatement of a material fact or omit to state a material fact necessary to make the statements therein, in light of the circumstances under which they were made, not misleading, and the Acquiror shall disseminate such correction to the stockholders of the Acquiror in an amendment or supplement and to cause such amendment or supplement to be filed with the SEC. The Acquiror shall notify the Company promptly in writing upon the receipt of any comments from the SEC and of any request by the SEC for amendments or supplements to the Information Statement and shall promptly supply the Company with copies of all such comments, requests and any other written correspondence between the Acquiror or any of its Representatives, on the one hand, and the SEC, on the other hand, with respect to the Information Statement. The Acquiror shall use its reasonable best efforts to respond as promptly as reasonably practicable to any comments received from the SEC concerning the Information Statement and to resolve such comments with the SEC and cause the Definitive Information Statement to be filed with the SEC as contemplated by Rule 14c-5 under the Exchange Act.
Annex A-39
(c) The Acquiror shall use its reasonable best efforts to cause the Definitive Information Statement to be disseminated to the Acquiror Stockholders as promptly as reasonably practicable after the first to occur of (A) confirmation from the SEC that it has no further comments on the Information Statement, (B) confirmation from the SEC that the Information Statement is otherwise not to be reviewed or (C) expiration of the 10-day period after filing the preliminary Information Statement in the event the SEC does not review the Information Statement. If the Acquiror, Merger Sub or any of their respective affiliates is required to file any other document with the SEC in connection with this Agreement or the Transactions, Acquiror shall provide the Company with a reasonable opportunity to review and to propose comments on any such document, which Acquiror shall consider in good faith.
Section 8.03 Tax Matters.
(a) Transfer Taxes. Except as otherwise set forth in this Agreement, all transfer, documentary, sales, use, stamp, registration, value added or other similar Taxes incurred in connection with the Transactions (“Transfer Taxes”) shall be borne 100% by the Company. The Company and Acquiror further agree to reasonably cooperate to reduce or eliminate the amount of any such Transfer Taxes.
(b) Tax Treatment. The parties intend that, for United States federal income tax purposes, subject to the last sentence of this Section 8.03(b), the Merger shall be treated as a sale of the Existing Company Common Stock by each holder of such shares of common stock in exchange for Acquiror Common Stock and Acquiror Preferred Stock. (collectively, the “Intended Tax Treatment”). The Transactions shall be reported by the parties for all Tax purposes in accordance with the Intended Tax Treatment, unless otherwise required by a Tax Authority as a result of a “determination” within the meaning of Section 1313(a) of the Code (or any similar or corresponding provision of applicable Law). The parties hereto shall, and shall cause their Affiliates to, cooperate with each other and their respective counsel to document and support the Intended Tax Treatment and, following the Closing, the parties hereto shall not, or and shall not permit or cause their respective controlled Affiliates to, take any action, or knowingly fail to take any action, which action or failure to act prevents or impedes, or would reasonably be expected to prevent or impede, the Transactions from qualifying for the Intended Tax Treatment. The Parties agree, that, at the option of the Acquiror, elections under Section 338(g) of the Code (and any analogous applicable state, local or non-U.S. election) may be made with respect to the purchase by Acquiror of the Existing Company Common Stock and the deemed purchase of the shares of TechyTrade FZ LLC or, alternatively, elections pursuant to U.S. Treasury Regulation Section 301.7701-3(c) may be made effective prior to the Closing Date to treat each of the Company and TechyTrade FZ LLC as an entity that is not classified as an association for U.S. federal income tax purposes.
Section 8.04 Confidentiality; Publicity.
(a) Each party agrees that it will, and will cause its respective Affiliates and Representatives to, hold in strict confidence and agrees that it will not, and will cause its respective Affiliates and Representatives not to disclose or use any Confidential Information. If a party is requested or required pursuant to written or oral questions or requests for information or documents in any litigation, Governmental Order, interrogatory, civil investigation, demand or other similar process to disclose any Confidential Information, then such party will notify the other promptly of the request or requirement so that the non-requesting party may seek an appropriate protective order or waive compliance with the provisions of this Section 8.04(a). If, in the absence of a protective order or the receipt of a waiver hereunder, a party is, on the advice of counsel, compelled to disclose any Confidential Information to any Governmental Authority or else stand liable for contempt, then such party may disclose the Confidential Information to the Governmental Authority; provided, however, that such party shall use it’s reasonable best efforts to obtain, at the request of the other, an order or other assurance that confidential treatment will be accorded to such portion of the Confidential Information required to be disclosed as the non-requesting party shall designate. The foregoing provisions shall not apply to any Confidential Information that is generally available to the public immediately prior to the time of disclosure unless such Confidential Information is so available due to the actions of a party.
(b) The initial Press Release relating to this Agreement shall be a joint press release, the text of which has been agreed to by each of Acquiror and the Company (the “Press Release”). Promptly after the issuance of the Press Release, Acquiror shall file a current report on Form 8-K (the “Form 8-K”) with the Press Release and a description of this Agreement as required by applicable Securities Laws, which the Company shall review, comment upon and approve (which approval shall not be unreasonably withheld, conditioned or delayed) prior to filing (with the Company reviewing, commenting upon and approving such Form 8-K in any event no later than the third (3rd) Business Day after the execution of this Agreement). In connection with the preparation of the Press Release, the Form 8-K, or any other report, statement, filing notice or application made by or on behalf of a party to any Governmental
Annex A-40
Authority or other third party in connection with the transactions contemplated hereby, each party shall, upon request by any other party, furnish the parties with all information concerning themselves, their respective directors, officers and equity holders, and such other matters as may be reasonably necessary or advisable in connection with the Transactions contemplated hereby, or any other report, statement, filing, notice or application made by or on behalf of a party to any third party and/or any Governmental Authority in connection with the Transactions contemplated hereby. Furthermore, nothing contained in this Section 8.04(b) shall prevent Acquiror or the Company or its respective Affiliates from furnishing customary or other reasonable information concerning the Transactions to their investors and prospective investors that is substantively consistent with public statements previously consented to by the other party in accordance with this Section 8.04(b).
Section 8.05 Notification of Certain Matters. During the Interim Period, each party will give prompt notice to the other parties if such party or its Affiliates: (a) fails to comply with or satisfy any covenant, condition or agreement to be complied with or satisfied by it or its Affiliates in any material respect; (b) receives any notice or other communication in writing from any third party (including any Governmental Authority) alleging (i) that the consent of such third party is or may be required in connection with the transactions contemplated by this Agreement or (ii) any non-compliance with any Law by such party or its Affiliates; (c) receives any notice or other communication from any Governmental Authority in connection with the transactions contemplated by this Agreement; or (d) becomes aware of the commencement or threat, in writing, of any Action against such party or any of its Affiliates, or any of their respective properties or assets, or, to the Knowledge of such party, any officer, director, partner, member or manager, in his, her or its capacity as such, of such party or of its Affiliates with respect to the consummation of the transactions contemplated by this Agreement. No such notice will constitute an acknowledgement or admission by the party providing the notice regarding whether or not any of the representations, warranties or covenants contained in this Agreement have been breached.
Section 8.06 Post-Closing Cooperation. Following the Closing, each party shall, on the request of any other party, execute such further documents, and perform such further acts, as may be reasonably necessary or appropriate to give full effect to the allocation of rights, benefits, obligations and liabilities contemplated by this Agreement and the transactions contemplated hereby.
Article IX
INTENTIONALLY OMITTED.
Article X
MISCELLANEOUS
Section 10.01 Notices. All notices and other communications among the parties shall be in writing and shall be deemed to have been duly given (i) when delivered in person, (ii) when delivered after posting in the United States mail having been sent registered or certified mail return receipt requested, postage prepaid, (iii) when delivered by FedEx or other nationally recognized overnight delivery service or (iv) when e-mailed during normal business hours (and otherwise as of the immediately following Business Day), addressed as follows:
(a) If to Acquiror or Merger Sub to:
Apimeds Pharmaceuticals US, Inc.
100 Matawan Road, Suite 325
New Jersey, NJ 07747
Attn: Erik Emerson
E-mail: erik@apimedsus.com
with a copy (which shall not constitute notice) to:
Nelson Mullins Riley & Scarborough LLP
301 Hillsborough Street, Suite 1400
Raleigh, NC 27603
Attn: David Mannheim; Mike Bradshaw
E-mail: david.mannheim@nelsonmullins.com; mike.bradshaw@nelsonmullins.com
Annex A-41
(b) If to the Company to:
TechyTrade Innovations Pte Ltd
60 Paya Lebar Road.
#04-23
Singapore 409051
Attn: Vin Menon
E-mail: vin@mindwavedao.com
with copies (which shall not constitute notice) to:
Duane Morris LLP
901 New York Avenue N.W., Suite 700 East
Washington, D.C. 20001-4795
Attn: Andrew M. Tucker
E-mail: atucker@duanemorris.com
or to such other address or addresses as the parties may from time to time designate in writing.
Section 10.02 Assignment. No party hereto shall assign this Agreement or any part hereof without the prior written consent of the other parties. Subject to the foregoing, this Agreement shall be binding upon and inure to the benefit of the parties hereto and their respective permitted successors and assigns. Any attempted assignment in violation of the terms of this Section 10.03 shall be null and void, ab initio.
Section 10.03 Rights of Third Parties. Except as otherwise provided in Section 7.02, Section 7.08(b) and Section 10.14, this Agreement is exclusively for the benefit of the Company, and its respective successors and permitted assigns, with respect to the obligations of Acquiror and Merger Sub under this Agreement, and for the benefit of Acquiror and Merger Sub, and their respective successors and permitted assigns, with respect to the obligations of the Company under this Agreement, and this Agreement shall not be deemed to confer upon or give to any other third party any remedy, claim, liability, reimbursement, cause of action or other right.
Section 10.04 Expenses. Except as otherwise provided herein (including Section 8.03(a) and this Section 10.4), each party hereto shall bear its own Transaction Expenses in connection with this Agreement and the Transactions, including all fees and expenses of legal counsel, investment banks, brokers, finders and other representatives. In addition, except as otherwise provided herein, each party shall be solely responsible for any and all expenses, liability, and obligations arising from or relating to its own operations prior to the Closing, including but not limited to, tax liabilities, payroll, employee benefits, severance, accrued compensation, and any other employment-related liabilities.
Section 10.05 Governing Law. This Agreement, and all claims or causes of action based upon, arising out of, or related to this Agreement or the transactions contemplated hereby, shall be governed by, and construed in accordance with, the Laws of the State of Delaware, without giving effect to principles or rules of conflict of laws to the extent such principles or rules would require or permit the application of Laws of another jurisdiction.
Section 10.06 Captions; Counterparts. The captions in this Agreement are for convenience only and shall not be considered a part of or affect the construction or interpretation of any provision of this Agreement. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. The words “execution,” “signed,” “signature,” and words of like import in this Agreement or in any other certificate, agreement or document related to this Agreement shall include images of manually executed signatures transmitted by facsimile or other electronic format (including, without limitation, “pdf,” “tif” or “jpg”) and other electronic signatures (including, without limitation, DocuSign and AdobeSign). The use of electronic signatures and electronic records (including, without limitation, any contract or other record created, generated, sent, communicated, received, or stored by electronic means) shall be of the same legal effect, validity and enforceability as a manually executed signature or use of a paper-based record-keeping system to the fullest extent permitted by applicable law, including the Federal Electronic Signatures in Global and National Commerce Act, the Uniform Electronic Transactions Act and any other applicable law, including, without limitation, any state law based on the Uniform Electronic Transactions Act or the Uniform Commercial Code.
Annex A-42
Section 10.07 Schedules and Exhibits. The Schedules and Exhibits referenced herein are a part of this Agreement as if fully set forth herein. All references herein to Schedules and Exhibits shall be deemed references to such parts of this Agreement, unless the context shall otherwise require. Any disclosure made by a party in the Schedules with reference to any section or schedule of this Agreement shall be deemed to be a disclosure with respect to all other sections or schedules to which such disclosure may apply solely to the extent the relevance of such disclosure is reasonably apparent on the face of the disclosure in such Schedule. Certain information set forth in the Schedules is included solely for informational purposes.
Section 10.08 Entire Agreement. This Agreement (together with the Schedules and Exhibits to this Agreement), and the Acquiror Support Agreement executed on the date hereof constitute the entire agreement among the parties relating to the transactions contemplated hereby and supersede any other agreements, whether written or oral, that may have been made or entered into by or among any of the parties hereto or any of their respective Subsidiaries relating to the transactions contemplated hereby. No representations, warranties, covenants, understandings, agreements, oral or otherwise, relating to the transactions contemplated by this Agreement exist between the parties except as expressly set forth or referenced in this Agreement.
Section 10.09 Amendments. This Agreement may be amended or modified in whole or in part, only by a duly authorized agreement in writing executed in the same manner as this Agreement (but not necessarily by the same natural persons who executed this Agreement) and which makes reference to this Agreement. The approval of this Agreement by the equityholders of any of the parties shall not restrict the ability of the board of directors of any of the parties to enter into an amendment to this Agreement pursuant to this Section 10.10.
Section 10.10 Severability. If any provision of this Agreement is held invalid or unenforceable by any court of competent jurisdiction, the other provisions of this Agreement shall remain in full force and effect. The parties further agree that if any provision contained herein is, to any extent, held invalid or unenforceable in any respect under the Laws governing this Agreement, they shall take any actions necessary to render the remaining provisions of this Agreement valid and enforceable to the fullest extent permitted by Law and shall amend or otherwise modify this Agreement to replace any provision contained herein that is held invalid or unenforceable with a valid and enforceable provision giving effect to the intent of the parties.
Section 10.11 Jurisdiction; WAIVER OF TRIAL BY JURY. Any Action based upon, arising out of or related to this Agreement, or the transactions contemplated hereby, shall be brought in any state or federal court located in the State of New York, and each of the parties irrevocably submits to the exclusive jurisdiction of each such court in any such Action, waives any objection it may now or hereafter have to personal jurisdiction, venue or to convenience of forum, agrees that all claims in respect of the Action shall be heard and determined only in any such court, and agrees not to bring any Action arising out of or relating to this Agreement or the transactions contemplated hereby in any other court. Nothing herein contained shall be deemed to affect the right of any party to serve process in any manner permitted by Law, or to commence legal proceedings or otherwise proceed against any other party in any other jurisdiction, in each case, to enforce judgments obtained in any Action brought pursuant to this Section 10.12. EACH OF THE PARTIES HERETO HEREBY IRREVOCABLY AND UNCONDITIONALLY WAIVES TO THE FULLEST EXTENT PERMITTED BY LAW ANY AND ALL RIGHT TO TRIAL BY JURY IN ANY ACTION BASED UPON, ARISING OUT OF OR RELATED TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY. EACH OF THE PARTIES HERETO CERTIFIES AND ACKNOWLEDGES THAT (I) NO REPRESENTATIVE OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH OTHER PARTY WOULD NOT, IN THE EVENT OF LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER, (II) EACH SUCH PARTY UNDERSTANDS AND HAS CONSIDERED THE IMPLICATIONS OF THIS WAIVER, (III) EACH SUCH PARTY MAKES THIS WAIVER VOLUNTARILY, AND (IV) EACH SUCH PARTY HAS BEEN INDUCED TO ENTER INTO THIS AGREEMENT AND THE TRANSACTIONS BY, AMONG OTHER THINGS, THE MUTUAL WAIVERS IN THIS SECTION 10.12.
Section 10.12 Enforcement. The parties agree that irreparable damage for which monetary damages, even if available, would not be an adequate remedy, would occur in the event that the parties do not perform their obligations under the provisions of this Agreement (including failing to take such actions as are required of them hereunder to consummate this Agreement) in accordance with its specified terms or otherwise breach such provisions. The parties acknowledge and agree that (a) the parties shall be entitled to an injunction, specific performance, or other equitable relief, to prevent breaches of this Agreement and to enforce specifically the terms and provisions hereof, without proof of damages, prior to the valid termination of this Agreement in accordance with Section 10.01, this being in addition
Annex A-43
to any other remedy to which they are entitled under this Agreement, and (b) the right of specific enforcement is an integral part of the transactions contemplated by this Agreement and without that right, none of the parties would have entered into this Agreement. Each party agrees that it will not oppose the granting of specific performance and other equitable relief on the basis that the other parties have an adequate remedy at Law or that an award of specific performance is not an appropriate remedy for any reason at Law or equity. The parties acknowledge and agree that any party seeking an injunction to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement in accordance with this Section 10.12 shall not be required to provide any bond or other security in connection with any such injunction.
Section 10.13 Non-Recourse. This Agreement may only be enforced against, and any claim or cause of action based upon, arising out of, or related to this Agreement or the transactions contemplated hereby may only be brought against, the entities that are expressly named as parties hereto, and then only with respect to the specific obligations set forth herein with respect to such party. Except to the extent a named party to this Agreement (and then only to the extent of the specific obligations undertaken by such named party in this Agreement), (a) no past, present or future director, manager, officer, employee, incorporator, member, partner, stockholder, Affiliate, agent, attorney, advisor or Representative or Affiliate of any named party to this Agreement and (b) no past, present or future director, manager, officer, employee, incorporator, member, partner, stockholder, Affiliate, agent, attorney, advisor or Representative or Affiliate of any of the foregoing shall have any liability (whether in contract, tort, equity or otherwise) for any one or more of the representations, warranties, covenants, agreements or other obligations or liabilities of any one or more of the Company, Acquiror or Merger Sub under this Agreement of or for any claim based on, arising out of, or related to this Agreement or the transactions contemplated hereby.
Section 10.14 Non-survival of Representations, Warranties and Covenants. None of the representations, warranties, covenants, obligations or other agreements in this Agreement or in any certificate, statement or instrument delivered pursuant to this Agreement, including any rights arising out of any breach of such representations, warranties, covenants, obligations, agreements and other provisions, shall survive the Closing and shall terminate and expire upon the occurrence of the Closing (and there shall be no liability after the Effective Time in respect thereof), except for (a) those covenants and agreements contained herein that by their terms expressly apply in whole or in part after the Closing and then only with respect to any breaches occurring after the Closing and (b) this Article X.
Section 10.15 Acknowledgements. Each of the parties acknowledges and agrees (on its own behalf and on behalf of its respective Affiliates and its and their respective Representatives) that: (a) it has conducted its own independent investigation of the financial condition, results of operations, assets, liabilities, properties and projected operations of the other parties (and their respective Subsidiaries) and has been afforded satisfactory access to the books and records, facilities and personnel of the other parties (and their respective Subsidiaries) for purposes of conducting such investigation; (b) the Company Representations constitute the sole and exclusive representations and warranties of the Company in connection with the transactions contemplated hereby; (c) the Acquiror and Merger Sub Representations constitute the sole and exclusive representations and warranties of Acquiror and Merger Sub; (d) except for the Company Representations by the Company and the Acquiror and Merger Sub Representations by each of Acquiror and Merger Sub, respectively, none of the parties hereto or any other Person makes, or has made, any other express or implied representation or warranty with respect to any party hereto (or any party’s Affiliates) or the transactions contemplated by this Agreement and all other representations and warranties of any kind or nature expressed or implied (including (i) regarding the completeness or accuracy of, or any omission to state or to disclose, any information, including in the estimates, projections or forecasts or any other information, document or material provided to or made available to any party hereto or their respective Affiliates or Representatives in certain “data rooms,” management presentations or in any other form in expectation of the Transactions, including meetings, calls or correspondence with management of any party hereto (or any party’s Subsidiaries), and (ii) any relating to the future or historical business, condition (financial or otherwise), results of operations, prospects, assets or liabilities of any party hereto (or its Subsidiaries), or the quality, quantity or condition of any party’s or its Subsidiaries’ assets) are specifically disclaimed by all parties hereto and their respective Subsidiaries and all other Persons (including the Representatives and Affiliates of any party hereto or its Subsidiaries); and (e) each party hereto and its respective Affiliates are not relying on any representations and warranties in connection with the Transactions except the Company Representations by the Company, the Acquiror and Merger Sub Representations by each of Acquiror and Merger Sub and the other representations expressly made by a Person in the Acquiror Support Agreement.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
Annex A-44
IN WITNESS WHEREOF, Acquiror, Merger Sub, Bio Sub, Bio Sub Representative, and the Company have caused this Agreement to be executed and delivered as of the date first written above by their respective officers thereunto duly authorized.
|
APIMEDS PHARMACEUTICALS US, INC. |
||||
|
By: |
/s/ Erik Emerson |
|||
|
Name: |
Erik Emerson |
|||
|
Title: |
Chief Executive Officer |
|||
[Signature Page to Agreement and Plan of Merger]
Annex A-45
|
APIMEDS MERGER SUB, INC. |
||||
|
By: |
/s/ Erik Emerson |
|||
|
Name: |
Erik Emerson |
|||
|
Title: |
Chief Executive Officer |
|||
[Signature Page to Agreement and Plan of Merger]
Annex A-46
|
MindWave Innovations Inc |
||||
|
By: |
/s/ Vin Menon |
|||
|
Name: |
Vin Menon |
|||
|
Title: |
Chief Executive Officer |
|||
[Signature Page to Agreement and Plan of Merger]
Annex A-47
|
Lokahi Therapeutics, Inc. |
||||
|
By: |
/s/ Erik Emerson |
|||
|
Name: |
Erik Emerson |
|||
|
Title: |
Chief Executive Officer |
|||
[Signature Page to Agreement and Plan of Merger]
Annex A-48
|
ERIK EMERSON, AS THE BIO BUSINESS REPRESENTATIVE |
||||
|
By: |
/s/ Erik Emerson |
|||
|
Name: |
Erik Emerson |
|||
[Signature Page to Agreement and Plan of Merger]
Annex A-49
SECURITIES PURCHASE AGREEMENT
This SECURITIES PURCHASE AGREEMENT (the “Agreement”), dated as of December 1, 2025, is by and among Apimeds Pharmaceuticals US, Inc., a Delaware corporation with offices located at 100 Matawan Road, Suite 325, New Jersey, NJ 07747 (the “Company”), and each of the investors listed on the Schedule of Buyers attached hereto (individually, a “Buyer” and collectively, the “Buyers”).
RECITALS
A. On December 1, 2025, the Company entered into that certain Agreement and Plan of Merger (as in effect as of the date hereof, the “Merger Agreement”), with MindWave Innovations Inc, a Delaware corporation (the “Target”), Apimeds Merger Sub, Inc., a Delaware corporation (“Merger Sub”), Lokahi Therapeutics, Inc., a Nevada corporation (“Bio Sub”), and Erik Emerson, solely in his capacity as representative for the Bio Business (the “Bio Business Representative”), pursuant to which, among other things, the Merger Sub shall merge with and into Target (the “Merger” and the other transactions contemplated by the Merger Agreement, the “Transactions”) and, at the closing thereof (the “Transaction Closing”, and such date, the “Transaction Closing Date”), the Target, as the surviving entity, shall be a wholly-owned subsidiary of the Company.
B. The Company and each Buyer is executing and delivering this Agreement in reliance upon the exemption from securities registration afforded by Section 4(a)(2) of the Securities Act of 1933, as amended (the “1933 Act”), and Rule 506(b) of Regulation D (“Regulation D”) as promulgated by the United States Securities and Exchange Commission (the “SEC”) under the 1933 Act.
C. The Company has authorized a new series of senior convertible notes of the Company, in the aggregate original principal amount of up to $120,900,000 in aggregate principal amount of senior convertible notes, substantially in the form attached hereto as Exhibit A (the “Notes”), which Notes shall be convertible into shares of Common Stock (as defined below) (the shares of Common Stock issuable pursuant to the terms of the Notes, including, without limitation, upon conversion, as Pre-Delivery Shares (as defined in the Notes) or otherwise, collectively, “Conversion Shares”), in accordance with the terms of the Notes.
D. Each Buyer wishes to purchase, and the Company wishes to sell at the Initial Closing (as defined below), upon the terms and conditions stated in this Agreement, a Note in the aggregate original principal amount as set forth opposite such Buyer’s name in column (3) on the Schedule of Buyers (which aggregate principal amount for all Buyers shall not exceed $10,875,000) (each an “Initial Note”, and collectively, the “Initial Notes”)(the Conversion Shares issuable pursuant to the terms of the Initial Notes, including, without limitation, upon conversion or otherwise, collectively, the “Initial Conversion Shares”).
E. Subject to the terms and conditions set forth in this Agreement, each Buyer, severally, may require the Company to participate in one or more Additional Optional Closings (as defined below) for the purchase by such Buyer, and the sale by the Company, of one or more Notes with an aggregate original principal amount for all Additional Optional Closings not to exceed the maximum aggregate principal amount as set forth opposite such Buyer’s name in column (4) on the Schedule of Buyers (which aggregate principal amount for all Buyers for all Additional Optional Closings shall not exceed $13,075,000 (or such other amount as the Company and each Buyer shall mutually agree in writing)) (each an “Additional Optional Note”, and collectively, the “Additional Optional Notes”); provided, that, subject to the satisfaction of the conditions below, the Company may force each Buyer to purchase the maximum aggregate principal amount of Additional Optional Notes as set forth opposite such Buyer’s name in column (5) on the Schedule of Buyers (which aggregate principal amount for all Buyers for all Additional Mandatory Closings (as defined below) shall not exceed $2,175,000 (or such other amount as the Company and each Buyer shall mutually agree in writing))(each an “Additional Mandatory Note”, and collectively, the “Additional Mandatory Notes”).
F. Subject to the terms and conditions set forth in this Agreement, the Company and each Buyer may mutually agree to consummate one or more Additional Mutual Closings (as defined below) for the purchase by such Buyer, and the sale by the Company, of one or more Notes with an aggregate original principal amount for all Additional Mutual Closings not to exceed such maximum aggregate principal amount of Notes as set forth in column (6) of the Schedule of Buyers (which aggregate principal amount for all Buyers for all Additional Mutual Closings shall not exceed $96,950,000 (or such other amount as the Company and each Buyer shall mutually agree in writing)) (each an “Additional Mutual Note”, and collectively, the “Additional Mutual Notes”, and together with the Additional
Annex B-1
Optional Notes and the Additional Mandatory Notes, collectively, the “Additional Notes”, and the Conversion Shares issuable pursuant to the terms of the Additional Notes, including, without limitation, upon conversion or otherwise, collectively, the “Additional Conversion Shares”).
G. At the Initial Closing, the parties hereto shall execute and deliver a Registration Rights Agreement, in the form attached hereto as Exhibit B (the “Registration Rights Agreement”), pursuant to which the Company has agreed to provide certain registration rights with respect to the Registrable Securities (as defined in the Registration Rights Agreement), under the 1933 Act and the rules and regulations promulgated thereunder, and applicable state securities laws.
H. The Notes and the Conversion Shares are collectively referred to herein as the “Securities.”
AGREEMENT
NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Company and each Buyer hereby agree as follows:
1. PURCHASE AND SALE OF NOTES.
(a) Purchase of Notes.
(i) Purchase of Initial Notes. Subject to the satisfaction (or waiver) of the conditions set forth in Sections 6(a) and 7(a) below, the Company shall issue and sell to each Buyer, and each Buyer severally, but not jointly, agrees to purchase from the Company on the Initial Closing Date (as defined below) an Initial Note in the original principal amount as is set forth opposite such Buyer’s name in column (3) on the Schedule of Buyers (the “Initial Closing”).
(ii) Purchase of Additional Notes. Subject to the satisfaction (or waiver) of the conditions set forth in Sections 1(b)(ii), 6(b) and 7(b) below, the Company shall issue and sell to such Buyer, and such Buyer severally, but not jointly, agrees to purchase from the Company certain Additional Notes on the applicable Additional Closing Date (as defined below) in such aggregate original principal amount as is set forth in such applicable Additional Closing Notice (as defined below) (each such closing of the purchase of such Additional Notes, each, an “Additional Closing”).
(b) Closing. Each of the Initial Closing and any Additional Closings (collectively, the “Closings”) of the purchase of the Notes by the Buyers shall occur at the offices of Kelley Drye & Warren LLP, 3 World Trade Center, 175 Greenwich Street, New York, NY 10007 (or at such other time and place as is mutually agreed upon between the parties).
(i) Initial Closing. The date and time of the Initial Closing (the “Initial Closing Date”) shall be 10:00 a.m., New York time, on the first (1st) Business Day on which the conditions to the Initial Closing set forth in Sections 6(a) and 7(a) below are satisfied or waived (or such other date as is mutually agreed to by the Company and each Buyer). As used herein “Business Day” means any day other than Saturday, Sunday or other day on which commercial banks in The City of New York are authorized or required by law to remain closed; provided, however, for clarification, commercial banks shall not be deemed to be authorized or required by law to remain closed due to “stay at home”, “shelter-in-place”, “non-essential employee” or any other similar orders or restrictions or the closure of any physical branch locations at the direction of any governmental authority so long as the electronic funds transfer systems (including for wire transfers) of commercial banks in The City of New York generally are open for use by customers on such day.
(ii) Additional Closings at the Company’s Election.
(1) General. Subject to the satisfaction (or waiver) of the conditions to closing set forth in this Section 1(b) with respect to such applicable Additional Closing and Section 6(b) and Section 7(b) below (Sections 6(b) and 7(b), collectively, the “Additional Closing Conditions”), on or after the first date on which the resale by the Buyers of the Required Registration Amount (as defined in the Registration Rights Agreement) of Registrable Securities underlying the Initial Notes and the Additional Notes issuable pursuant to this Section 1(b)(ii) is registered pursuant to a Registration Statement (as defined in the Registration Rights Agreement) of the Company declared effective by
Annex B-2
the SEC (and each prospectus contained therein is available for use by each applicable Buyer on such date) (the “Additional Mandatory Closing Eligibility Condition”), the Company shall have the right exercisable by delivery to the Buyers of one or more written notices (each an “Additional Mandatory Closing Notice”, and the date of each Additional Mandatory Closing Notice, each, an “Additional Mandatory Closing Notice Date”), executed by the chief executive officer or chief financial officer of the Company, to require each Buyer to purchase at an Additional Closing (the “Additional Mandatory Closing”, and such date, the “Additional Mandatory Closing Date”), subject to the satisfaction (or waiver) of the Additional Closing Conditions of an Additional Note in the aggregate original principal amount as set forth opposite such Buyer’s name in column (5) on the Schedule of Buyers (which aggregate principal amount for all Buyers shall not exceed $2,175,000) (subject to reduction, on a dollar for dollar basis to an amount not less than zero (0), for any Additional Optional Notes issued in any Additional Optional Closings prior to such date of determination). Each Additional Mandatory Closing Notice shall (A) certify that the Additional Mandatory Closing Eligibility Condition has been satisfied as of such date of determination and that, other than with respect to deliverables to be delivered to each Buyer at such Additional Mandatory Closing, all of the Additional Closing Conditions have been satisfied in full as of such applicable Additional Mandatory Closing Notice Date, (B) state the aggregate principal amount of the Additional Notes to be purchased by the Buyers (which, with respect to any given Buyer shall not exceed such aggregate principal amount of such Additional Notes as set forth opposite its name in column (4) on the Schedule of Buyers and (C) specify the proposed date of such Additional Mandatory Closing (which shall be no less than two (2) Business Days nor more than five (5) Business Days after such Additional Mandatory Closing Notice Date, subject to the right of each Buyer, by written notice to the Company, to accelerate such applicable Additional Closing Date (each, an “Additional Mandatory Closing Date”) to an earlier date, not less than one (1) Trading Day after such applicable Additional Mandatory Closing Notice Date (or such other date as such Buyer and the Company shall mutually agree)), and (E) confirm whether or not such Additional Mandatory Closing Notice constitutes material non-public information. Each Additional Mandatory Closing Notice shall be irrevocable, and the Company may only deliver one Additional Mandatory Closing Notice. For the avoidance of doubt, the Buyers shall not be required to consummate any Additional Closing if on the Additional Closing Date the Company fails to satisfy the Additional Mandatory Closing Notice Eligibility Condition, if an Event of Default exists or if the Company fails to satisfy any of the other conditions to closing herein (unless waived in writing by the applicable Buyer participating in such Additional Mandatory Closing). The Company’s right to require a Buyer to purchase Additional Mandatory Notes pursuant to an Additional Mandatory Closing Notice shall automatically expire at 9:00 AM EST on the earlier of (x) such date as all Additional Notes issuable hereunder have been issued hereunder and (y) the tenth (10th) Trading Day after the Additional Mandatory Closing Eligibility Condition is initially satisfied.
(iii) Additional Closings at Buyers Election. Subject to the satisfaction (or waiver) of the Additional Closing Conditions, at any time after the Initial Closing Date, each Buyer, severally, shall have the right, exercisable by e-mail delivery of a written notice to the Company (each, an “Additional Optional Closing Notice”, and the date thereof, each an “Additional Closing Notice Date”) to purchase, and to require the Company to sell to such Buyer, at one or more Additional Closings (each such Closing, an “Additional Optional Closing”, and the date of any such Additional Optional Closing, each, an “Additional Optional Closing Date”), up to such maximum aggregate principal amount of Additional Optional Notes equal to the amount as set forth opposite such Buyer’s name in column (5) on the Schedule of Buyers (subject to reduction, on a dollar-for-dollar basis, for the aggregate principal amount of any Additional Mandatory Notes issued in any Additional Mandatory Closing prior to such applicable Additional Mandatory Closing Date)(each, an “Additional Closing Maximum Amount”). Each Additional Optional Closing Notice shall specify (x) the proposed date and time of the applicable Additional Closing (which, if unspecified in such Additional Optional Closing Notice, shall be the first (1st) Trading Day (as defined in the Notes) after such Additional Optional Closing Notice or such other date as is mutually agreed to by the Company and each Buyer) and (y) the aggregate principal amount of Additional Notes to be purchased by each Buyer at such applicable Additional Optional Closing, which shall not exceed the Additional Closing Maximum Amount of such
Annex B-3
applicable Buyer (or such other amount as the Company and such Buyer shall mutually agree). The Buyers’ rights to effect any Additional Optional Closings hereunder shall terminate at 9:00 AM EST on the earlier of (x) such date as all Additional Notes issuable hereunder have been issued hereunder and (y) the 24 month anniversary of the Initial Closing Date (the “Additional Closing Expiration Date”).
(iv) Additional Mutual Closings. At any time after the Initial Closing Date, the Company or any Buyer, severally (as applicable, the “Initiating Party”) may deliver one or more written notices (each, an “Additional Mutual Closing Notice”, and together with each Additional Mandatory Closing Notice, Additional Optional Closing Notice and each Additional Mandatory Closing Notice, each an “Additional Closing Notice”, and the date of each Additional Mutual Closing Notice, each, an “Additional Mutual Closing Notice Date”, and together with each Additional Mandatory Closing Notice Date, each Additional Buyer Optional Closing Notice Date and each Additional Mandatory Closing Notice Date, each an “Additional Closing Notice Date”) to the other party (the “Responding Party”), (A) requesting an Additional Closing (each, an “Additional Mutual Closing”, and together with the Additional Optional Closings and the Additional Mandatory Closing, each an “Additional Closing”, and the date of any such Additional Mutual Closing, each an “Additional Mutual Closing Date”, and together with each Additional Optional Closing Date and Additional Mandatory Closing Date, each an “Additional Closing Date”) of such aggregate principal amount of the Additional Notes to be purchased by such applicable Buyer as set forth in such Additional Mutual Closing Notice (which, together with the aggregate principal amount of any Additional Notes issued at any prior Additional Mutual Closings, shall not exceed the maximum aggregate principal amount as set forth opposite such Buyer’s name in column (6) on the Schedule of Buyers), and (B) setting forth the proposed Additional Mutual Closing Date. If a Responding Party fails to execute and return such Additional Mutual Closing Notice to the Initiating Party within five (5) Business Days of receipt, such Additional Mutual Closing Notice shall be automatically cancelled. For the avoidance of doubt, no Additional Mutual Closing shall occur hereunder unless both the Company and each such applicable Buyer shall have duly executed and delivered an Additional Mutual Closing Notice with respect thereto and no party shall be under any obligation to execute and deliver any Additional Mutual Closing Notice. Notwithstanding anything herein to the contrary, no Additional Mutual Closings shall occur hereunder from and after the Additional Closing Expiration Date
(c) Purchase Price. The aggregate purchase price for the Initial Notes to be purchased by each Buyer (the “Initial Purchase Price”) shall be the amount set forth opposite such Buyer’s name in column (7) on the Schedule of Buyers. The aggregate purchase price for the Additional Notes to be purchased by each Buyer at any given Additional Closing (each, an “Additional Purchase Price”, and together with the Initial Purchase Price, each, a “Purchase Price”) which together with the Additional Purchase Price of each prior Additional Closing, shall not exceed the aggregate amount set forth opposite such Buyer’s name in column (8) of the Schedule of Buyers. The aggregate purchase price for the Notes to be purchased by each Buyer at any given Closing shall be $919.54 for each $1,000 of aggregate principal amount of Notes.
(d) Form of Payment.
(i) Initial Closing. On the Initial Closing Date, (i) each Buyer shall pay its respective Initial Purchase Price (less, in the case of any Buyer, the amounts withheld pursuant to Section 4(g)) to the Company for the Initial Notes to be issued and sold to such Buyer at the Initial Closing, by wire transfer of immediately available funds in accordance with the Initial Flow of Funds Letter (as defined below) and (ii) the Company shall deliver to each Buyer an Initial Note in the aggregate original principal amount as is set forth opposite such Buyer’s name in column (3) of the Schedule of Buyers, duly executed on behalf of the Company and registered in the name of such Buyer or its designee.
(ii) Additional Closing. On each Additional Closing Date, (i) each Buyer shall pay its respective applicable Additional Purchase Price for such Additional Closing (less, in the case of any Buyer, the amounts withheld pursuant to Section 4(g)) to the Company for the Additional Notes to be issued and sold to such Buyer at each Additional Closing, by wire transfer of immediately available funds in accordance with the applicable Additional Flow of Funds Letter (as defined below) and (ii) the Company shall deliver to each Buyer an Additional Note in the aggregate original principal amount as is set forth in the applicable Additional Closing Notice to be issued to such Buyer, duly executed on behalf of the Company and registered in the name of such Buyer or its designee.
Annex B-4
2. BUYER’S REPRESENTATIONS AND WARRANTIES.
Each Buyer, severally and not jointly, represents and warrants to the Company with respect to only itself that, as of the date hereof and as of each Closing Date:
(a) Organization; Authority. Such Buyer is an entity duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization with the requisite power and authority to enter into and to consummate the transactions contemplated by the Transaction Documents (as defined below) to which it is a party and otherwise to carry out its obligations hereunder and thereunder.
(b) No Public Sale or Distribution. Such Buyer (i) is acquiring its Note, and (ii) upon conversion of its Note will acquire the Conversion Shares issuable upon conversion thereof, in each case, for its own account and not with a view towards, or for resale in connection with, the public sale or distribution thereof in violation of applicable securities laws, except pursuant to sales registered or exempted under the 1933 Act; provided, however, by making the representations herein, such Buyer does not agree, or make any representation or warranty, to hold any of the Securities for any minimum or other specific term and reserves the right to dispose of the Securities at any time in accordance with or pursuant to a registration statement or an exemption from registration under the 1933 Act. Such Buyer does not presently have any agreement or understanding, directly or indirectly, with any Person to distribute any of the Securities in violation of applicable securities laws. For purposes of this Agreement, “Person” means an individual, a limited liability company, a partnership, a joint venture, a corporation, a trust, an unincorporated organization, any other entity and any Governmental Entity (as defined below) or any department or agency thereof.
(c) Accredited Investor Status. Such Buyer is an “accredited investor” as that term is defined in Rule 501(a) of Regulation D.
(d) Reliance on Exemptions. Such Buyer understands that the Securities are being offered and sold to it in reliance on specific exemptions from the registration requirements of United States federal and state securities laws and that the Company is relying in part upon the truth and accuracy of, and such Buyer’s compliance with, the representations, warranties, agreements, acknowledgments and understandings of such Buyer set forth herein in order to determine the availability of such exemptions and the eligibility of such Buyer to acquire the Securities.
(e) Information. Such Buyer and its advisors, if any, have been furnished with all materials relating to the business, finances and operations of the Company and materials relating to the offer and sale of the Securities that have been requested by such Buyer. Such Buyer and its advisors, if any, have been afforded the opportunity to ask questions of the Company. Neither such inquiries nor any other due diligence investigations conducted by such Buyer or its advisors, if any, or its representatives shall modify, amend or affect such Buyer’s right to rely on the Company’s representations and warranties contained herein. Such Buyer understands that its investment in the Securities involves a high degree of risk. Such Buyer has sought such accounting, legal and tax advice as it has considered necessary to make an informed investment decision with respect to its acquisition of the Securities.
(f) No Governmental Review. Such Buyer understands that no United States federal or state agency or any other government or governmental agency has passed on or made any recommendation or endorsement of the Securities or the fairness or suitability of the investment in the Securities nor have such authorities passed upon or endorsed the merits of the offering of the Securities.
(g) Transfer or Resale. Such Buyer understands that except as provided in the Registration Rights Agreement and Section 4(h) hereof: (i) the Securities have not been and are not being registered under the 1933 Act or any state securities laws, and may not be offered for sale, sold, assigned or transferred unless (A) subsequently registered thereunder, (B) such Buyer shall have delivered to the Company (if requested by the Company) an opinion of counsel, in a form reasonably acceptable to the Company, to the effect that such Securities to be sold, assigned or transferred may be sold, assigned or transferred pursuant to an exemption from such registration, or (C) such Buyer provides the Company with reasonable assurance that such Securities can be sold, assigned or transferred pursuant to Rule 144 or Rule 144A promulgated under the 1933 Act (or a successor rule thereto) (collectively, “Rule 144”); (ii) any sale of the Securities made in reliance on Rule 144 may be made only in accordance with the terms of Rule 144, and further, if Rule 144 is not applicable, any resale of the Securities under circumstances in which the seller (or the Person through whom the sale is made) may be deemed to be an underwriter (as that term is defined in the 1933 Act) may require compliance with some other exemption under the 1933 Act or the rules and regulations of the SEC promulgated thereunder; and (iii) neither the Company nor any other Person is under any obligation to register the Securities under
Annex B-5
the 1933 Act or any state securities laws or to comply with the terms and conditions of any exemption thereunder. Notwithstanding the foregoing, the Securities may be pledged in connection with a bona fide margin account or other loan or financing arrangement secured by the Securities and such pledge of Securities shall not be deemed to be a transfer, sale or assignment of the Securities hereunder, and no Buyer effecting a pledge of Securities shall be required to provide the Company with any notice thereof or otherwise make any delivery to the Company pursuant to this Agreement or any other Transaction Document (as defined in Section 3(b)), including, without limitation, this Section 2(g).
(h) Validity; Enforcement. This Agreement and the Registration Rights Agreement have been duly and validly authorized, executed and delivered on behalf of such Buyer and shall constitute the legal, valid and binding obligations of such Buyer enforceable against such Buyer in accordance with their respective terms, except as such enforceability may be limited by general principles of equity or to applicable bankruptcy, insolvency, reorganization, moratorium, liquidation and other similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies.
(i) No Conflicts. The execution, delivery and performance by such Buyer of this Agreement and the Registration Rights Agreement and the consummation by such Buyer of the transactions contemplated hereby and thereby will not (i) result in a violation of the organizational documents of such Buyer, or (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which such Buyer is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws) applicable to such Buyer, except in the case of clauses (ii) and (iii) above, for such conflicts, defaults, rights or violations which could not, individually or in the aggregate, reasonably be expected to have a material adverse effect on the ability of such Buyer to perform its obligations hereunder.
(j) Residency. Such Buyer is a resident of (or organized under the laws of, as applicable) that jurisdiction specified below its address on the Schedule of Buyers.
3. REPRESENTATIONS AND WARRANTIES OF THE COMPANY.
The Company represents and warrants to each of the Buyers that, as of the date hereof and as of each Closing Date:
(a) Organization and Qualification. Each of the Company and each of its Subsidiaries (as defined below) are entities duly organized and validly existing and in good standing under the laws of the jurisdiction in which they are formed, and have the requisite power and authority to own their properties and to carry on their business as now being conducted and as presently proposed to be conducted. Each of the Company and each of its Subsidiaries is duly qualified as a foreign entity to do business and is in good standing in every jurisdiction in which its ownership of property or the nature of the business conducted by it makes such qualification necessary, except to the extent that the failure to be so qualified or be in good standing would not reasonably be expected to have a Material Adverse Effect (as defined below). As used in this Agreement, “Material Adverse Effect” means any material adverse effect on (i) the business, properties, assets, liabilities, operations (including results thereof), condition (financial or otherwise) or prospects of the Company or any Subsidiary, individually or taken as a whole, (ii) the transactions contemplated hereby or in any of the other Transaction Documents or any other agreements or instruments to be entered into in connection herewith or therewith or (iii) the authority or ability of the Company or any of its Subsidiaries to perform any of their respective obligations under any of the Transaction Documents (as defined below). Other than the Persons (as defined below) set forth on Schedule 3(a), the Company has no Subsidiaries. “Subsidiaries” means any Person in which the Company, directly or indirectly, (I) owns any of the outstanding capital stock or holds any equity or similar interest of such Person or (II) controls or operates all or any part of the business, operations or administration of such Person, and each of the foregoing, is individually referred to herein as a “Subsidiary.”
(b) Authorization; Enforcement; Validity. The Company has the requisite power and authority to enter into and perform its obligations under this Agreement and the other Transaction Documents and to issue the Securities in accordance with the terms hereof and thereof. The execution and delivery of this Agreement and the other Transaction Documents by the Company and its Subsidiaries, and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the Notes and the reservation for issuance and issuance of the Conversion Shares issuable upon conversion of the Notes) have been duly authorized by the Company’s board of directors or other governing body, as applicable, and (other than the filing of (i) with the SEC
Annex B-6
of one or more Registration Statements in accordance with the requirements of the Registration Rights Agreement, (ii) a Listing of Additional Shares application with the Principal Market, (iii) the Stockholder Approval (as defined below), (iv) a Form D with the SEC and (v) any other filings as may be required by any state securities agencies (collectively, the “Required Approvals”)) no further filing, consent or authorization is required by the Company, its Subsidiaries, their respective boards of directors or their stockholders or other governing body. This Agreement has been, and the other Transaction Documents to which it is a party will be prior to such Closing, duly executed and delivered by the Company, and each constitutes the legal, valid and binding obligations of the Company, enforceable against the Company in accordance with its respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies and except as rights to indemnification and to contribution may be limited by federal or state securities law. “Transaction Documents” means, collectively, this Agreement, the Notes, the Registration Rights Agreement, the Voting Agreements (as defined below), the Irrevocable Transfer Agent Instructions (as defined below) and each of the other agreements and instruments entered into or delivered by any of the parties hereto in connection with the transactions contemplated hereby and thereby, as may be amended from time to time.
(c) Issuance of Securities. The issuance of the Notes is duly authorized and upon issuance in accordance with the terms of the Transaction Documents shall be validly issued, fully paid and non-assessable and free from all preemptive or similar rights, mortgages, defects, claims, liens, pledges, charges, taxes, rights of first refusal, encumbrances, security interests and other encumbrances (collectively “Liens”) with respect to the issuance thereof. As of the Initial Closing, the Company shall have reserved from its duly authorized capital stock not less than the Required Reserve Amount (as defined below). Upon issuance or conversion in accordance with the Notes, the Conversion Shares, when issued, will be validly issued, fully paid and nonassessable and free from all preemptive or similar rights or Liens with respect to the issue thereof, with the holders being entitled to all rights accorded to a holder of Common Stock. Subject to the accuracy of the representations and warranties of the Buyers in this Agreement, the offer and issuance by the Company of the Securities is exempt from registration under the 1933 Act.
(d) No Conflicts. The execution, delivery and performance of the Transaction Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the Notes and the Conversion Shares and the reservation for issuance of the Conversion Shares) will not (i) result in a violation of the Certificate of Incorporation (as defined below) (including, without limitation, any certificate of designation contained therein), Bylaws (as defined below), certificate of formation, memorandum of association, articles of association, bylaws or other organizational documents of the Company or any of its Subsidiaries, or any capital stock or other securities of the Company or any of its Subsidiaries, (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) in any respect under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Company or any of its Subsidiaries is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including, without limitation, foreign, federal and state securities laws and regulations and the rules and regulations of NYSE American LLC (the “Principal Market”) and including all applicable foreign, federal and state laws, rules and regulations) applicable to the Company or any of its Subsidiaries or by which any property or asset of the Company or any of its Subsidiaries is bound or affected.
(e) Consents. Neither the Company nor any Subsidiary is required to obtain any consent from, authorization or order of, or make any filing or registration with (other than the Required Approvals), any Governmental Entity (as defined below) or any regulatory or self-regulatory agency or any other Person in order for it to execute, deliver or perform any of its respective obligations under or contemplated by the Transaction Documents, in each case, in accordance with the terms hereof or thereof. All consents, authorizations, orders, filings and registrations which the Company or any Subsidiary is required to obtain pursuant to the preceding sentence have been or will be obtained or effected on or prior to the applicable Closing Date, and neither the Company nor any of its Subsidiaries are aware of any facts or circumstances which might prevent the Company or any of its Subsidiaries from obtaining or effecting any of the registration, application or filings contemplated by the Transaction Documents. The Company is not in violation of the requirements of the Principal Market and has no knowledge of any facts or circumstances which could reasonably lead to delisting or suspension of the Common Stock in the foreseeable future. “Governmental Entity” means any nation, state, county, city, town, village, district, or other political jurisdiction of any nature, federal, state, local, municipal, foreign, or other government, governmental or quasi-governmental authority of any nature (including any governmental agency, branch, department, official, or entity and any court or other tribunal), multi-national
Annex B-7
organization or body; or body exercising, or entitled to exercise, any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power of any nature or instrumentality of any of the foregoing, including any entity or enterprise owned or controlled by a government or a public international organization or any of the foregoing.
(f) Acknowledgment Regarding Buyer’s Purchase of Securities. The Company acknowledges and agrees that each Buyer is acting solely in the capacity of an arm’s length purchaser with respect to the Transaction Documents and the transactions contemplated hereby and thereby and that no Buyer is (i) an officer or director of the Company or any of its Subsidiaries, (ii) an “affiliate” (as defined in Rule 144) of the Company or any of its Subsidiaries or (iii) to its knowledge, a “beneficial owner” of more than 10% of the shares of Common Stock (as defined for purposes of Rule 13d-3 of the Securities Exchange Act of 1934, as amended (the “1934 Act”)). The Company further acknowledges that no Buyer is acting as a financial advisor or fiduciary of the Company or any of its Subsidiaries (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated hereby and thereby, and any advice given by a Buyer or any of its representatives or agents in connection with the Transaction Documents and the transactions contemplated hereby and thereby is merely incidental to such Buyer’s purchase of the Securities. The Company further represents to each Buyer that the Company’s and each Subsidiary’s decision to enter into the Transaction Documents to which it is a party has been based solely on the independent evaluation by the Company, each Subsidiary and their respective representatives.
(g) No General Solicitation; Placement Agent’s Fees. Neither the Company, nor any of its Subsidiaries or affiliates, nor any Person acting on its or their behalf, has engaged in any form of general solicitation or general advertising (within the meaning of Regulation D) in connection with the offer or sale of the Securities. The Company shall be responsible for the payment of any placement agent’s fees, financial advisory fees, or brokers’ commissions (other than for Persons engaged by any Buyer or its investment advisor) relating to or arising out of the transactions contemplated hereby, including, without limitation, placement agent fees payable to E.F. Hutton & Co. LLC, as placement agent (the “Placement Agent”) in connection with the sale of the Securities. The fees and expenses of the Placement Agent to be paid by the Company or any of its Subsidiaries are as set forth on Schedule 3(g) attached hereto. The Company shall pay, and hold each Buyer harmless against, any liability, loss or expense (including, without limitation, attorney’s fees and out-of-pocket expenses) arising in connection with any such claim. The Company acknowledges that it has engaged the Placement Agent in connection with the sale of the Securities. Other than the Placement Agent, neither the Company nor any of its Subsidiaries has engaged any placement agent or other agent in connection with the offer or sale of the Securities.
(h) No Integrated Offering. None of the Company, its Subsidiaries or any of their affiliates, nor any Person acting on their behalf has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would require registration of the issuance of any of the Securities under the 1933 Act, whether through integration with prior offerings or otherwise, or cause this offering of the Securities to require approval of stockholders of the Company for purposes of the 1933 Act or under any applicable stockholder approval provisions, including, without limitation, under the rules and regulations of the Principal Market or any other exchange or automated quotation system on which any of the securities of the Company are listed or designated for quotation. None of the Company, its Subsidiaries, their affiliates nor any Person acting on their behalf will take any action or steps that would require registration of the issuance of any of the Securities under the 1933 Act or cause the offering of any of the Securities to be integrated with other offerings of securities of the Company.
(i) Dilutive Effect. The Company understands and acknowledges that the number of Conversion Shares will increase in certain circumstances. The Company further acknowledges that its obligation to issue the Conversion Shares pursuant to the terms of the Notes in accordance with this Agreement and the Notes are, in each case, absolute and unconditional regardless of the dilutive effect that such issuance may have on the ownership interests of other stockholders of the Company.
(j) Application of Takeover Protections; Rights Agreement. The Company and its board of directors have taken all necessary action, if any, in order to render inapplicable any control share acquisition, interested stockholder, business combination, poison pill (including, without limitation, any distribution under a rights agreement), stockholder rights plan or other similar anti-takeover provision under the Certificate of Incorporation, Bylaws or other organizational documents or the laws of the jurisdiction of its incorporation or otherwise which is or could become applicable to any Buyer as a result of the transactions contemplated by this Agreement, including, without limitation, the Company’s issuance of the Securities and any Buyer’s ownership of the Securities. The Company and its board of directors have taken all necessary action, if any, in order to render inapplicable any stockholder rights plan or similar arrangement relating to accumulations of beneficial ownership of shares of Common Stock or a change in control of the Company or any of its Subsidiaries.
Annex B-8
(k) SEC Documents; Financial Statements. During the two (2) years prior to the date hereof (or such shorter time that the Company has been required to file such reports), the Company has timely filed all reports, schedules, forms, proxy statements, statements and other documents required to be filed by it with the SEC pursuant to the reporting requirements of the 1934 Act (all of the foregoing filed prior to the date hereof and all exhibits and appendices included therein and financial statements, notes and schedules thereto and documents incorporated by reference therein being hereinafter referred to as the “SEC Documents”). The Company has delivered or has made available to the Buyers or their respective representatives true, correct and complete copies of each of the SEC Documents not available on the EDGAR system. As of their respective dates, the SEC Documents complied in all material respects with the requirements of the 1934 Act and the rules and regulations of the SEC promulgated thereunder applicable to the SEC Documents, and none of the SEC Documents, at the time they were filed with the SEC, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. As of their respective dates, the financial statements of the Company included in the SEC Documents complied in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto as in effect as of the time of filing. Such financial statements have been prepared in accordance with generally accepted accounting principles (“GAAP”), consistently applied, during the periods involved (except (i) as may be otherwise indicated in such financial statements or the notes thereto, or (ii) in the case of unaudited interim statements, to the extent they may exclude footnotes or may be condensed or summary statements) and fairly present in all material respects the financial position of the Company as of the dates thereof and the results of its operations and cash flows for the periods then ended (subject, in the case of unaudited statements, to normal year-end audit adjustments which will not be material, either individually or in the aggregate). The reserves, if any, established by the Company or the lack of reserves, if applicable, are reasonable based upon facts and circumstances known by the Company on the date hereof and there are no loss contingencies that are required to be accrued by the Statement of Financial Accounting Standard No. 5 of the Financial Accounting Standards Board which are not provided for by the Company in its financial statements or otherwise. No other information provided by or on behalf of the Company to any of the Buyers which is not included in the SEC Documents (including, without limitation, information referred to in Section 2(e) of this Agreement or in the disclosure schedules to this Agreement) contains any untrue statement of a material fact or omits to state any material fact necessary in order to make the statements therein not misleading, in the light of the circumstance under which they are or were made. The Company is not currently contemplating to amend or restate any of the financial statements (including, without limitation, any notes or any letter of the independent accountants of the Company with respect thereto) included in the SEC Documents (the “Financial Statements”), nor is the Company currently aware of facts or circumstances which would require the Company to amend or restate any of the Financial Statements, in each case, in order for any of the Financial Statements to be in compliance with GAAP and the rules and regulations of the SEC. The Company has not been informed by its independent accountants that they recommend that the Company amend or restate any of the Financial Statements or that there is any need for the Company to amend or restate any of the Financial Statements.
(l) Absence of Certain Changes. Since the date of the Company’s most recent audited financial statements contained in a Form 10-K, there has been no material adverse change and no material adverse development in the business, assets, liabilities, properties, operations (including results thereof), condition (financial or otherwise) or prospects of the Company or any of its Subsidiaries other than the Transactions. Since the date of the Company’s most recent audited financial statements contained in a Form 10-K, neither the Company nor any of its Subsidiaries has (i) declared or paid any dividends, (ii) sold any assets, individually or in the aggregate, outside of the ordinary course of business or (iii) made any capital expenditures, individually or in the aggregate, outside of the ordinary course of business. Neither the Company nor any of its Subsidiaries has taken any steps to seek protection pursuant to any law or statute relating to bankruptcy, insolvency, reorganization, receivership, liquidation or winding up, nor does the Company or any Subsidiary have any knowledge or reason to believe that any of their respective creditors intend to initiate involuntary bankruptcy proceedings or any actual knowledge of any fact which would reasonably lead a creditor to do so. The Company and its Subsidiaries, individually and on a consolidated basis, are not as of the date hereof, and after giving effect to the transactions contemplated hereby to occur at such Closing, will not be Insolvent (as defined below). For purposes of this Section 3(l), “Insolvent” means, (i) with respect to the Company and its Subsidiaries, on a consolidated basis, (A) the present fair saleable value of the Company’s and its Subsidiaries’ assets is less than the amount required to pay the Company’s and its Subsidiaries’ total Indebtedness (as defined below), (B) the Company and its Subsidiaries are unable to pay their debts and liabilities, subordinated, contingent or otherwise, as such debts and liabilities become absolute and matured or (C) the Company and its Subsidiaries intend to incur or believe that they will incur debts that would be beyond their ability to pay as such debts mature; and (ii) with
Annex B-9
respect to the Company and each Subsidiary, individually, (A) the present fair saleable value of the Company’s or such Subsidiary’s (as the case may be) assets is less than the amount required to pay its respective total Indebtedness, (B) the Company or such Subsidiary (as the case may be) is unable to pay its respective debts and liabilities, subordinated, contingent or otherwise, as such debts and liabilities become absolute and matured or (C) the Company or such Subsidiary (as the case may be) intends to incur or believes that it will incur debts that would be beyond its respective ability to pay as such debts mature. Neither the Company nor any of its Subsidiaries has engaged in any business or in any transaction, and is not about to engage in any business or in any transaction, for which the Company’s or such Subsidiary’s remaining assets constitute unreasonably small capital with which to conduct the business in which it is engaged as such business is now conducted and is proposed to be conducted.
(m) No Undisclosed Events, Liabilities, Developments or Circumstances. No event, liability, development or circumstance has occurred or exists, or is reasonably expected to exist or occur with respect to the Company, any of its Subsidiaries or any of their respective businesses, properties, liabilities, prospects, operations (including results thereof) or condition (financial or otherwise), that (i) would be required to be disclosed by the Company under applicable securities laws on a registration statement on Form S-1 filed with the SEC relating to an issuance and sale by the Company of its Common Stock and which has not been publicly announced, (ii) could have a material adverse effect on any Buyer’s investment hereunder or (iii) could have a Material Adverse Effect.
(n) Conduct of Business; Regulatory Permits. Neither the Company nor any of its Subsidiaries is in violation of any term of or in default under its Certificate of Incorporation, any certificate of designation, preferences or rights of any other outstanding series of preferred stock of the Company or any of its Subsidiaries or Bylaws or their organizational charter, certificate of formation, memorandum of association, articles of association, Certificate of Incorporation or certificate of incorporation or bylaws, respectively. Neither the Company nor any of its Subsidiaries is in violation of any judgment, decree or order or any statute, ordinance, rule or regulation applicable to the Company or any of its Subsidiaries, and neither the Company nor any of its Subsidiaries will conduct its business in violation of any of the foregoing, except in all cases for possible violations which could not, individually or in the aggregate, have a Material Adverse Effect. Without limiting the generality of the foregoing, the Company is not in violation of any of the rules, regulations or requirements of the Principal Market and has no knowledge of any facts or circumstances that could reasonably lead to delisting or suspension of the Common Stock by the Principal Market in the foreseeable future. During the two years prior to the date hereof (or such shorter time as may be applicable), (i) the Common Stock has been listed or designated for quotation on the Principal Market, (ii) trading in the Common Stock has not been suspended by the SEC or the Principal Market and (iii) the Company has received no communication, written or oral, from the SEC or the Principal Market regarding the suspension or delisting of the Common Stock from the Principal Market. The Company and each of its Subsidiaries possess all certificates, authorizations and permits issued by the appropriate regulatory authorities necessary to conduct their respective businesses, except where the failure to possess such certificates, authorizations or permits would not have, individually or in the aggregate, a Material Adverse Effect, and neither the Company nor any such Subsidiary has received any notice of proceedings relating to the revocation or modification of any such certificate, authorization or permit. There is no agreement, commitment, judgment, injunction, order or decree binding upon the Company or any of its Subsidiaries or to which the Company or any of its Subsidiaries is a party which has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of the Company or any of its Subsidiaries, any acquisition of property by the Company or any of its Subsidiaries or the conduct of business by the Company or any of its Subsidiaries as currently conducted other than such effects, individually or in the aggregate, which have not had and would not reasonably be expected to have a Material Adverse Effect on the Company or any of its Subsidiaries.
(o) Foreign Corrupt Practices. Neither the Company, the Company’s subsidiary or any director, officer, agent, employee, nor any other person acting for or on behalf of the foregoing (individually and collectively, a “Company Affiliate”) have violated the U.S. Foreign Corrupt Practices Act (the “FCPA”) or any other applicable anti-bribery or anti-corruption laws, nor has any Company Affiliate offered, paid, promised to pay, or authorized the payment of any money, or offered, given, promised to give, or authorized the giving of anything of value, to any officer, employee or any other person acting in an official capacity for any Governmental Entity to any political party or official thereof or
Annex B-10
to any candidate for political office (individually and collectively, a “Government Official”) or to any person under circumstances where such Company Affiliate knew or was aware of a high probability that all or a portion of such money or thing of value would be offered, given or promised, directly or indirectly, to any Government Official, for the purpose of:
(i) (A) influencing any act or decision of such Government Official in his/her official capacity, (B) inducing such Government Official to do or omit to do any act in violation of his/her lawful duty, (C) securing any improper advantage, or (D) inducing such Government Official to influence or affect any act or decision of any Governmental Entity, or
(ii) assisting the Company or its Subsidiaries in obtaining or retaining business for or with, or directing business to, the Company or its Subsidiaries.
(p) Sarbanes-Oxley Act. The Company and each Subsidiary is in compliance with any and all applicable requirements of the Sarbanes-Oxley Act of 2002, as amended, and any and all applicable rules and regulations promulgated by the SEC thereunder.
(q) Transactions With Affiliates. Except as disclosed in the SEC Documents, no current or former employee, partner, director, officer or stockholder (direct or indirect) of the Company or its Subsidiaries, or any associate, or, to the knowledge of the Company, any affiliate of any thereof, or any relative with a relationship no more remote than first cousin of any of the foregoing, is presently, or has ever been, (i) a party to any transaction with the Company or its Subsidiaries (including any contract, agreement or other arrangement providing for the furnishing of services by, or rental of real or personal property from, or otherwise requiring payments to, any such director, officer or stockholder or such associate or affiliate or relative Subsidiaries (other than for ordinary course services as employees, officers or directors of the Company or any of its Subsidiaries)) or (ii) the direct or indirect owner of an interest in any corporation, firm, association or business organization which is a competitor, supplier or customer of the Company or its Subsidiaries (except for a passive investment (direct or indirect) in less than 5% of the common stock of a company whose securities are traded on or quoted through an Eligible Market (as defined below)), nor does any such Person receive income from any source other than the Company or its Subsidiaries which relates to the business of the Company or its Subsidiaries or should properly accrue to the Company or its Subsidiaries. No employee, officer, stockholder or director of the Company or any of its Subsidiaries or member of his or her immediate family is indebted to the Company or its Subsidiaries, as the case may be, nor is the Company or any of its Subsidiaries indebted (or committed to make loans or extend or guarantee credit) to any of them, other than (i) for payment of salary for services rendered, (ii) reimbursement for reasonable expenses incurred on behalf of the Company, and (iii) for other standard employee benefits made generally available to all employees or executives (including stock option agreements outstanding under any stock option plan approved by the Board of Directors of the Company).
(r) Equity Capitalization.
(i) Definitions:
(A) “Common Stock” means (x) the Company’s shares of common stock, $0.01 par value per share, and (y) any capital stock into which such common stock shall have been changed or any share capital resulting from a reclassification of such common stock.
(B) “Preferred Stock” means (x) the Company’s blank check preferred stock, $0.01 par value per share, the terms of which may be designated by the board of directors of the Company in a certificate of designations and (y) any capital stock into which such preferred stock shall have been changed or any share capital resulting from a reclassification of such preferred stock (other than a conversion of such preferred stock into Common Stock in accordance with the terms of such certificate of designations).
(ii) Authorized and Outstanding Capital Stock. As of the date hereof, the authorized capital stock of the Company consists of (A) 100,000,000 shares of Common Stock, of which, 16,839,007 are issued and outstanding and 2,394,501 shares are reserved for issuance pursuant to Common Stock Equivalents (as defined below) (other than the Notes) exercisable or exchangeable for, or convertible into, shares of Common Stock and (B) 6,907,817 shares of Preferred Stock, all of which are issued and outstanding. No
Annex B-11
shares of Common Stock are held in the treasury of the Company. “Common Stock Equivalents” means any capital stock or other security of the Company or any of its Subsidiaries that is at any time and under any circumstances directly or indirectly convertible into, exercisable or exchangeable for, or which otherwise entitles the holder thereof to acquire, any capital stock or other security of the Company (including, without limitation, Common Stock) or any of its Subsidiaries. After giving effect to the Transaction Closing (including without limitation the consummation of any reverse stock split contemplated thereby), the Company will have at least 100,000,000 shares of Common Stock authorized.
(iii) Valid Issuance; Available Shares; Affiliates. All of such outstanding shares are duly authorized and have been, or upon issuance will be, validly issued and are fully paid and nonassessable. Schedule 3(r)(iii) sets forth the number of shares of Common Stock that are (A) reserved for issuance pursuant to Common Stock Equivalents (other than the Notes) and (B) that are, as of the date hereof, owned by Persons who are “affiliates” (as defined in Rule 405 of the 1933 Act and calculated based on the assumption that only officers, directors and holders of at least 10% of the Company’s issued and outstanding Common Stock are “affiliates” without conceding that any such Persons are “affiliates” for purposes of federal securities laws) of the Company or any of its Subsidiaries. Except as set forth on Schedule 3(r)(iii), no the Company’s knowledge, no Person owns 10% or more of the Company’s issued and outstanding shares of Common Stock (calculated based on the assumption that all Common Stock Equivalents (as defined below), whether or not presently exercisable or convertible, have been fully exercised or converted (as the case may be) taking account of any limitations on exercise or conversion (including “blockers”) contained therein without conceding that such identified Person is a 10% stockholder for purposes of federal securities laws).
(iv) Existing Securities; Obligations. Except as disclosed in the SEC Documents: (A) none of the Company’s or any Subsidiary’s assets. shares, interests or capital stock is subject to preemptive rights or any other similar rights or Liens suffered or permitted by the Company or any Subsidiary; (B) there are no outstanding options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible into, or exercisable or exchangeable for, any shares, interests or capital stock of the Company or any of its Subsidiaries, or contracts, commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to issue additional shares, interests or capital stock of the Company or any of its Subsidiaries or options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible into, or exercisable or exchangeable for, any shares, interests or capital stock of the Company or any of its Subsidiaries; (C) there are no agreements or arrangements under which the Company or any of its Subsidiaries is obligated to register the sale of any of their securities under the 1933 Act (except pursuant to the Registration Rights Agreement); (D) there are no outstanding securities or instruments of the Company or any of its Subsidiaries which contain any redemption or similar provisions, and there are no contracts, commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to redeem a security of the Company or any of its Subsidiaries; (E) there are no securities or instruments containing anti-dilution or similar provisions that will be triggered by the issuance of the Securities; and (F) neither the Company nor any Subsidiary has any stock appreciation rights or “phantom stock” plans or agreements or any similar plan or agreement.
(v) Organizational Documents. The Company has furnished to the Buyers true, correct and complete copies of the Company’s Amended and Restated Certificate of Incorporation, as amended and as in effect on the date hereof (the “Certificate of Incorporation”), and the Company’s Amended and Restated Bylaws, as amended and as in effect on the date hereof (the “Bylaws”), and the terms of all Common Stock Equivalents and the material rights of the holders thereof in respect thereto.
(s) Indebtedness and Other Contracts. Neither the Company nor any of its Subsidiaries, (i) except as disclosed on Schedule 3(s), (i) has any outstanding debt securities, notes, credit agreements, credit facilities or other agreements, documents or instruments evidencing Indebtedness of the Company or any of its Subsidiaries or by which the Company or any of its Subsidiaries is or may become bound, (ii) is a party to any contract, agreement or instrument, the violation of which, or default under which, by the other party(ies) to such contract, agreement or instrument could reasonably be expected to result in a Material Adverse Effect, (iii) has any financing statements securing obligations in any amounts filed in connection with the Company or any of its Subsidiaries; (iv) is in violation of any term of, or in default under, any contract, agreement or instrument relating to any Indebtedness, except where such violations and defaults would not result, individually or in the aggregate, in a Material Adverse Effect, or (v) is a party to any contract, agreement or
Annex B-12
instrument relating to any Indebtedness, the performance of which, in the judgment of the Company’s officers, has or is expected to have a Material Adverse Effect. Neither the Company nor any of its Subsidiaries have any liabilities or obligations required to be disclosed in the SEC Documents which are not so disclosed in the SEC Documents, other than those incurred in the ordinary course of the Company’s or its Subsidiaries’ respective businesses and which, individually or in the aggregate, do not or could not have a Material Adverse Effect. For purposes of this Agreement: (x) “Indebtedness” of any Person means, without duplication (A) all indebtedness for borrowed money, (B) all obligations issued, undertaken or assumed as the deferred purchase price of property or services (including, without limitation, “capital leases” in accordance with GAAP) (other than trade payables entered into in the ordinary course of business consistent with past practice), (C) all reimbursement or payment obligations with respect to letters of credit, surety bonds and other similar instruments, (D) all obligations evidenced by notes, bonds, debentures or similar instruments, including obligations so evidenced incurred in connection with the acquisition of property, assets or businesses, (E) all indebtedness created or arising under any conditional sale or other title retention agreement, or incurred as financing, in either case with respect to any property or assets acquired with the proceeds of such indebtedness (even though the rights and remedies of the seller or bank under such agreement in the event of default are limited to repossession or sale of such property), (F) all monetary obligations under any leasing or similar arrangement which, in connection with GAAP, consistently applied for the periods covered thereby, is classified as a capital lease, (G) all indebtedness referred to in clauses (A) through (F) above secured by (or for which the holder of such Indebtedness has an existing right, contingent or otherwise, to be secured by) any Lien upon or in any property or assets (including accounts and contract rights) owned by any Person, even though the Person which owns such assets or property has not assumed or become liable for the payment of such indebtedness, and (H) all Contingent Obligations in respect of indebtedness or obligations of others of the kinds referred to in clauses (A) through (G) above; and (y) “Contingent Obligation” means, as to any Person, any direct or indirect liability, contingent or otherwise, of that Person with respect to any Indebtedness, lease, dividend or other obligation of another Person if the primary purpose or intent of the Person incurring such liability, or the primary effect thereof, is to provide assurance to the obligee of such liability that such liability will be paid or discharged, or that any agreements relating thereto will be complied with, or that the holders of such liability will be protected (in whole or in part) against loss with respect thereto.
(t) Litigation. There is no action, suit, arbitration, proceeding, inquiry or investigation before or by the Principal Market, any court, public board, other Governmental Entity, self-regulatory organization or body pending or, to the knowledge of the Company, threatened against or affecting the Company or any of its Subsidiaries, the Common Stock or any of the Company’s or its Subsidiaries’ officers or directors, whether of a civil or criminal nature or otherwise, in their capacities as such. No director, officer or employee of the Company or any of its subsidiaries has willfully violated 18 U.S.C. §1519 or engaged in spoliation in reasonable anticipation of litigation. Without limitation of the foregoing, there has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by the SEC involving the Company, any of its Subsidiaries or any current or former director or officer of the Company or any of its Subsidiaries. The SEC has not issued any stop order or other order suspending the effectiveness of any registration statement filed by the Company under the 1933 Act or the 1934 Act. After reasonable inquiry of its employees, the Company is not aware of any fact which might result in or form the basis for any such action, suit, arbitration, investigation, inquiry or other proceeding. Neither the Company nor any of its Subsidiaries is subject to any order, writ, judgment, injunction, decree, determination or award of any Governmental Entity.
(u) Insurance. The Company and each of its Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as management of the Company believes to be prudent and customary in the businesses in which the Company and its Subsidiaries are engaged. Neither the Company nor any such Subsidiary has been refused any insurance coverage sought or applied for, and neither the Company nor any such Subsidiary has any reason to believe that it will be unable to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business at a cost that would not have a Material Adverse Effect.
(v) Employee Relations. Neither the Company nor any of its Subsidiaries is a party to any collective bargaining agreement or employs any member of a union. The Company and its Subsidiaries believe that their relations with their employees are good. No current (or former) executive officer (as defined in Rule 501(f) promulgated under the 1933 Act) or other key employee of the Company or any of its Subsidiaries has notified the Company or any such Subsidiary that such officer intends to leave the Company or any such Subsidiary or otherwise terminate such officer’s employment with the Company or any such Subsidiary. No current (or former) executive officer or other key employee of the Company or any of its Subsidiaries is, or is now expected to be, in violation of any material term of any employment contract, confidentiality, disclosure or proprietary information agreement, non-competition
Annex B-13
agreement, or any other contract or agreement or any restrictive covenant, and the continued employment of each such executive officer or other key employee (as the case may be) does not subject the Company or any of its Subsidiaries to any liability with respect to any of the foregoing matters. The Company and its Subsidiaries are in compliance with all federal, state, local and foreign laws and regulations respecting labor, employment and employment practices and benefits, terms and conditions of employment and wages and hours, except where failure to be in compliance would not, either individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect.
(w) Title.
(i) Real Property. Each of the Company and its Subsidiaries holds good title to all real property, leases in real property, facilities or other interests in real property owned or held by the Company or any of its Subsidiaries (the “Real Property”) owned by the Company or any of its Subsidiaries (as applicable). The Real Property is free and clear of all Liens and is not subject to any rights of way, building use restrictions, exceptions, variances, reservations, or limitations of any nature except for (a) Liens for current taxes not yet due and (b) zoning laws and other land use restrictions that do not impair the present or anticipated use of the property subject thereto. Any Real Property held under lease by the Company or any of its Subsidiaries are held by them under valid, subsisting and enforceable leases with such exceptions as are not material and do not interfere with the use made and proposed to be made of such property and buildings by the Company or any of its Subsidiaries.
(ii) Fixtures and Equipment. Each of the Company and its Subsidiaries (as applicable) has good title to, or a valid leasehold interest in, the tangible personal property, equipment, improvements, fixtures, and other personal property and appurtenances that are used by the Company or its Subsidiary in connection with the conduct of its business (the “Fixtures and Equipment”). The Fixtures and Equipment are structurally sound, are in good operating condition and repair, are adequate for the uses to which they are being put, are not in need of maintenance or repairs except for ordinary, routine maintenance and repairs and are sufficient for the conduct of the Company’s and/or its Subsidiaries’ businesses (as applicable) in the manner as conducted prior to such Closing. Each of the Company and its Subsidiaries owns all of its Fixtures and Equipment free and clear of all Liens except for (a) liens for current taxes not yet due and (b) zoning laws and other land use restrictions that do not impair the present or anticipated use of the property subject thereto.
(iii) Bitcoin Holdings Ownership. The Company owns and holds 1000 Bitcoin (as defined below) as of the date hereof and such applicable Closing Date (the “Bitcoin Holdings”), which is held in its digital wallet with the bank or other financial institution set forth on Schedule 3(w)(iii) attached hereto. The Company is the lawful owner of the Bitcoin Holdings with good and marketable title thereto, free and clear of any and all Liens and the Company has the absolute right to sell, assign, convey, transfer and deliver the Bitcoin Holdings without restriction. The Bitcoin Holdings was acquired by the Company in an arms-length transaction (or mined by the Company or any of its Subsidiaries in the ordinary course of business) complying with all applicable laws, rules and/or regulations with respect thereto and neither the Company nor any of its Subsidiaries is aware of any illicit activity with respect thereto and/or the origin thereof. The purchase price for the Bitcoin Holdings, if any, has been fully paid by the Company. Neither the Company nor any of its Subsidiaries has, directly or indirectly, entered into any written or oral agreement to sell, assign, convey or transfer the Bitcoin Holdings. “Bitcoin” means one unit of the digital currency traded under the ticker symbol “BTC”.
(x) Intellectual Property Rights. The Company and its Subsidiaries own or possess adequate rights or licenses to use all trademarks, trade names, service marks, service mark registrations, service names, original works of authorship, patents, patent rights, copyrights, inventions, licenses, approvals, governmental authorizations, trade secrets and other intellectual property rights and all applications and registrations therefor (“Intellectual Property Rights”) necessary to conduct their respective businesses as now conducted and presently proposed to be conducted. Each of the patents owned by the Company or any of its Subsidiaries is listed on Schedule 3(x)(i). Except as set forth in Schedule 3(x)(ii), none of the Company’s Intellectual Property Rights have expired or terminated or have been abandoned or are expected to expire or terminate or are expected to be abandoned, within three years from the date of this Agreement. The Company does not have any knowledge of any infringement by the Company or its Subsidiaries of Intellectual Property Rights of others. There is no claim, action or proceeding being made or brought, or to the knowledge of the Company or any of its Subsidiaries, being threatened, against the Company or any of its Subsidiaries regarding its Intellectual Property Rights. Neither the Company nor any of its Subsidiaries is aware of any facts or
Annex B-14
circumstances which might give rise to any of the foregoing infringements or claims, actions or proceedings. The Company and its Subsidiaries have taken reasonable security measures to protect the secrecy, confidentiality and value of all of their Intellectual Property Rights.
(y) Environmental Laws(i). (i) The Company and its Subsidiaries (A) are in compliance with any and all Environmental Laws (as defined below), (B) have received all permits, licenses or other approvals required of them under applicable Environmental Laws to conduct their respective businesses and (C) are in compliance with all terms and conditions of any such permit, license or approval where, in each of the foregoing clauses (A), (B) and (C), the failure to so comply could be reasonably expected to have, individually or in the aggregate, a Material Adverse Effect. The term “Environmental Laws” means all federal, state, local or foreign laws relating to pollution or protection of human health or the environment (including, without limitation, ambient air, surface water, groundwater, land surface or subsurface strata), including, without limitation, laws relating to emissions, discharges, releases or threatened releases of chemicals, pollutants, contaminants, or toxic or hazardous substances or wastes (collectively, “Hazardous Materials”) into the environment, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials, as well as all authorizations, codes, decrees, demands or demand letters, injunctions, judgments, licenses, notices or notice letters, orders, permits, plans or regulations issued, entered, promulgated or approved thereunder.
(ii) No Hazardous Materials:
(A) have been disposed of or otherwise released from any Real Property of the Company or any of its Subsidiaries in violation of any Environmental Laws; or
(B) are present on, over, beneath, in or upon any Real Property or any portion thereof in quantities that would constitute a violation of any Environmental Laws. No prior use by the Company or any of its Subsidiaries of any Real Property has occurred that violates any Environmental Laws, which violation would have a material adverse effect on the business of the Company or any of its Subsidiaries.
(iii) Neither the Company nor any of its Subsidiaries knows of any other person who or entity which has stored, treated, recycled, disposed of or otherwise located on any Real Property any Hazardous Materials, including, without limitation, such substances as asbestos and polychlorinated biphenyls.
(iv) None of the Real Properties are on any federal or state “Superfund” list or Liability Information System (“CERCLIS”) list or any state environmental agency list of sites under consideration for CERCLIS, nor subject to any environmental related Liens.
(z) Subsidiary Rights. The Company or one of its Subsidiaries has the unrestricted right to vote, and (subject to limitations imposed by applicable law) to receive dividends and distributions on, all capital securities of its Subsidiaries as owned by the Company or such Subsidiary.
(aa) Tax Status. The Company and each of its Subsidiaries (i) has timely made or filed all foreign, federal and state income and all other tax returns, reports and declarations required by any jurisdiction to which it is subject, (ii) has timely paid all taxes and other governmental assessments and charges that are material in amount, shown or determined to be due on such returns, reports and declarations, except those being contested in good faith and (iii) has set aside on its books provision reasonably adequate for the payment of all taxes for periods subsequent to the periods to which such returns, reports or declarations apply. There are no unpaid taxes in any material amount claimed to be due by the taxing authority of any jurisdiction, and the officers of the Company and its Subsidiaries know of no basis for any such claim. The Company is not operated in such a manner as to qualify as a passive foreign investment company, as defined in Section 1297 of the Internal Revenue Code of 1986, as amended (the “Code”). The net operating loss carryforwards (“NOLs”) for United States federal income tax purposes of the consolidated group of which the Company is the common parent, if any, shall not be adversely effected by the transactions contemplated hereby. The transactions contemplated hereby do not constitute an “ownership change” within the meaning of Section 382 of the Code, thereby preserving the Company’s ability to utilize such NOLs.
(bb) Internal Accounting and Disclosure Controls. The Company and each of its Subsidiaries maintains internal control over financial reporting (as such term is defined in Rule 13a-15(f) under the 1934 Act) that is effective to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, including that (i) transactions
Annex B-15
are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with GAAP and to maintain asset and liability accountability, (iii) access to assets or incurrence of liabilities is permitted only in accordance with management’s general or specific authorization and (iv) the recorded accountability for assets and liabilities is compared with the existing assets and liabilities at reasonable intervals and appropriate action is taken with respect to any difference. The Company maintains disclosure controls and procedures (as such term is defined in Rule 13a-15(e) under the 1934 Act) that are effective in ensuring that information required to be disclosed by the Company in the reports that it files or submits under the 1934 Act is recorded, processed, summarized and reported, within the time periods specified in the rules and forms of the SEC, including, without limitation, controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the 1934 Act is accumulated and communicated to the Company’s management, including its principal executive officer or officers and its principal financial officer or officers, as appropriate, to allow timely decisions regarding required disclosure. Neither the Company nor any of its Subsidiaries has received any notice or correspondence from any accountant, Governmental Entity or other Person relating to any potential material weakness or significant deficiency in any part of the internal controls over financial reporting of the Company or any of its Subsidiaries.
(cc) Off Balance Sheet Arrangements. There is no transaction, arrangement, or other relationship between the Company or any of its Subsidiaries and an unconsolidated or other off balance sheet entity that is required to be disclosed by the Company in its 1934 Act filings and is not so disclosed or that otherwise could be reasonably likely to have a Material Adverse Effect.
(dd) Investment Company Status. The Company is not, and upon consummation of the sale of the Securities will not be, an “investment company,” an affiliate of an “investment company,” a company controlled by an “investment company” or an “affiliated person” of, or “promoter” or “principal underwriter” for, an “investment company” as such terms are defined in the Investment Company Act of 1940, as amended.
(ee) Acknowledgement Regarding Buyers’ Trading Activity. Subject to Section 4(z) below, it is understood and acknowledged by the Company that (i) following the public disclosure of the transactions contemplated by the Transaction Documents, in accordance with the terms thereof, none of the Buyers have been asked by the Company or any of its Subsidiaries to agree, nor has any Buyer agreed with the Company or any of its Subsidiaries, to desist from effecting any transactions in or with respect to (including, without limitation, purchasing or selling, long and/or short) any securities of the Company, or “derivative” securities based on securities issued by the Company or to hold any of the Securities for any specified term; (ii) any Buyer, and counterparties in “derivative” transactions to which any such Buyer is a party, directly or indirectly, presently may have a “short” position in the Common Stock which was established prior to such Buyer’s knowledge of the transactions contemplated by the Transaction Documents; (iii) each Buyer shall not be deemed to have any affiliation with or control over any arm’s length counterparty in any “derivative” transaction; and (iv) each Buyer may rely on the Company’s obligation to timely deliver shares of Common Stock upon conversion or exchange, as applicable, of the Securities as and when required pursuant to the Transaction Documents for purposes of effecting trading in the Common Stock of the Company. The Company further understands and acknowledges that following the public disclosure of the transactions contemplated by the Transaction Documents pursuant to the Initial 8-K Filing (as defined below), subject to Section 4(z) below, one or more Buyers may engage in hedging and/or trading activities (including, without limitation, the location and/or reservation of borrowable shares of Common Stock) at various times during the period that the Securities are outstanding, including, without limitation, during the periods that the value and/or number of the Conversion Shares deliverable with respect to the Securities are being determined and such hedging and/or trading activities (including, without limitation, the location and/or reservation of borrowable shares of Common Stock), if any, can reduce the value of the existing stockholders’ equity interest in the Company both at and after the time the hedging and/or trading activities are being conducted. The Company acknowledges that such aforementioned hedging and/or trading activities do not constitute a breach of this Agreement, the Notes or any other Transaction Document or any of the documents executed in connection herewith or therewith, subject to Section 4(z) below.
(ff) Manipulation of Price. Neither the Company nor any of its Subsidiaries has, and, to the knowledge of the Company, no Person acting on their behalf has, directly or indirectly, (i) taken any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company or any of its Subsidiaries to facilitate the sale or resale of any of the Securities, (ii) sold, bid for, purchased, or paid any compensation for soliciting purchases of, any of the Securities (other than the Placement Agent), (iii) paid or agreed to pay to any Person any compensation for soliciting another to purchase any other securities of the Company or any of its Subsidiaries or (iv) paid or agreed to pay any Person for research services with respect to any securities of the Company or any of its Subsidiaries.
Annex B-16
(gg) U.S. Real Property Holding Corporation. Neither the Company nor any of its Subsidiaries is, or has ever been, and so long as any of the Securities are held by any of the Buyers, shall become, a U.S. real property holding corporation within the meaning of Section 897 of the Code, and the Company and each Subsidiary shall so certify upon any Buyer’s request.
(hh) Registration Eligibility. The Company is eligible to register the Registrable Securities (defined in the Registration Rights Agreement) for resale by the Buyers using Form S-1 promulgated under the 1933 Act.
(ii) Transfer Taxes. On each Closing Date, all stock transfer or other taxes (other than income or similar taxes) which are required to be paid in connection with the issuance, sale and transfer of the Securities to be sold to each Buyer hereunder will be, or will have been, fully paid or provided for by the Company, and all laws imposing such taxes will be or will have been complied with.
(jj) Bank Holding Company Act; Regulation U or X.
(i) Neither the Company nor any of its Subsidiaries is subject to the Bank Holding Company Act of 1956, as amended (the “BHCA”) and to regulation by the Board of Governors of the Federal Reserve System of the United States (the “Federal Reserve”). Neither the Company nor any of its Subsidiaries or affiliates owns or controls, directly or indirectly, five percent (5%) or more of the outstanding shares of any class of voting securities or twenty-five percent (25%) or more of the total equity of a bank or any entity that is subject to the BHCA and to regulation by the Federal Reserve. Neither the Company nor any of its Subsidiaries or affiliates exercises a controlling influence over the management or policies of a bank or any entity that is subject to the BHCA and to regulation by the Federal Reserve.
(ii) The sale of the Notes, the use of proceeds thereof and the other transactions contemplated thereby or by the other Transaction Documents, will not violate or be inconsistent with the provisions of Regulation U or X of the Board of Governors of the Federal Reserve.
(kk) Illegal or Unauthorized Payments; Political Contributions. Neither the Company nor any of its Subsidiaries nor, to the best of the Company’s knowledge (after reasonable inquiry of its officers and directors), any of the officers, directors, employees, agents or other representatives of the Company or any of its Subsidiaries or any other business entity or enterprise with which the Company or any Subsidiary is or has been affiliated or associated, has, directly or indirectly, made or authorized any payment, contribution or gift of money, property, or services, whether or not in contravention of applicable law, (i) as a kickback or bribe to any Person or (ii) to any political organization, or the holder of or any aspirant to any elective or appointive public office except for personal political contributions not involving the direct or indirect use of funds of the Company or any of its Subsidiaries.
(ll) Money Laundering. The Company and its Subsidiaries are in compliance with, and have not previously violated, the USA Patriot Act of 2001 and all other applicable U.S. and non-U.S. anti-money laundering laws and regulations, including, without limitation, the laws, regulations and Executive Orders and sanctions programs administered by the U.S. Office of Foreign Assets Control, including, but not limited, to (i) Executive Order 13224 of September 23, 2001 entitled, “Blocking Property and Prohibiting Transactions With Persons Who Commit, Threaten to Commit, or Support Terrorism” (66 Fed. Reg. 49079 (2001)); and (ii) any regulations contained in 31 CFR, Subtitle B, Chapter V.
(mm) Management. Except as set forth in Schedule 3(mm) hereto, during the past five year period, no current or former officer or director or, to the knowledge of the Company, no current ten percent (10%) or greater stockholder of the Company or any of its Subsidiaries has been the subject of:
(i) a petition under bankruptcy laws or any other insolvency or moratorium law or the appointment by a court of a receiver, fiscal agent or similar officer for such Person, or any partnership in which such person was a general partner at or within two years before the filing of such petition or such appointment, or any corporation or business association of which such person was an executive officer at or within two years before the time of the filing of such petition or such appointment;
(ii) a conviction in a criminal proceeding or a named subject of a pending criminal proceeding (excluding traffic violations that do not relate to driving while intoxicated or driving under the influence);
Annex B-17
(iii) any order, judgment or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining any such person from, or otherwise limiting, the following activities:
(1) Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the United States Commodity Futures Trading Commission or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
(2) Engaging in any particular type of business practice; or
(3) Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of securities laws or commodities laws;
(iv) any order, judgment or decree, not subsequently reversed, suspended or vacated, of any authority barring, suspending or otherwise limiting for more than sixty (60) days the right of any such person to engage in any activity described in the preceding sub paragraph, or to be associated with persons engaged in any such activity;
(v) a finding by a court of competent jurisdiction in a civil action or by the SEC or other authority to have violated any securities law, regulation or decree and the judgment in such civil action or finding by the SEC or any other authority has not been subsequently reversed, suspended or vacated; or
(vi) a finding by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any federal commodities law, and the judgment in such civil action or finding has not been subsequently reversed, suspended or vacated.
(nn) Stock Option Plans(b). Each stock option granted by the Company was granted (i) in accordance with the terms of the applicable stock option plan of the Company and (ii) with an exercise price at least equal to the fair market value of the Common Stock on the date such stock option would be considered granted under GAAP and applicable law. No stock option granted under the Company’s stock option plan has been backdated. The Company has not knowingly granted, and there is no and has been no policy or practice of the Company to knowingly grant, stock options prior to, or otherwise knowingly coordinate the grant of stock options with, the release or other public announcement of material information regarding the Company or its Subsidiaries or their financial results or prospects.
(oo) No Disagreements with Accountants and Lawyers(c). There are no material disagreements of any kind presently existing, or reasonably anticipated by the Company to arise, between the Company and the accountants and lawyers formerly or presently employed by the Company and the Company is current with respect to any fees owed to its accountants and lawyers which could affect the Company’s ability to perform any of its obligations under any of the Transaction Documents. In addition, on or prior to the date hereof, the Company had discussions with its accountants about its financial statements previously filed with the SEC. Based on those discussions, the Company has no reason to believe that it will need to restate any such financial statements or any part thereof.
(pp) No Disqualification Events(d). With respect to Securities to be offered and sold hereunder in reliance on Rule 506(b) under the 1933 Act (“Regulation D Securities”), none of the Company, any of its predecessors, any affiliated issuer, any director, executive officer, other officer of the Company participating in the offering contemplated hereby, any beneficial owner of 20% or more of the Company’s outstanding voting equity securities, calculated on the basis of voting power, nor any promoter (as that term is defined in Rule 405 under the 1933 Act) connected with the Company in any capacity at the time of sale (each, an “Issuer Covered Person” and, together, “Issuer Covered Persons”) is subject to any of the “Bad Actor” disqualifications described in Rule 506(d)(1)(i) to (viii) under the 1933 Act (a “Disqualification Event”), except for a Disqualification Event covered by Rule 506(d)(2) or (d)(3). The Company has exercised reasonable care to determine whether any Issuer Covered Person is subject to a Disqualification Event. The Company has complied, to the extent applicable, with its disclosure obligations under Rule 506(e), and has furnished to the Buyers a copy of any disclosures provided thereunder.
Annex B-18
(qq) Other Covered Persons(e). The Company is not aware of any Person (other than the Placement Agent) that has been or will be paid (directly or indirectly) remuneration for solicitation of Buyers or potential purchasers in connection with the sale of any Regulation D Securities.
(rr) No Additional Agreements. The Company does not have any agreement or understanding with any Buyer with respect to the transactions contemplated by the Transaction Documents other than as specified in the Transaction Documents.
(ss) Public Utility Holding Act. None of the Company nor any of its Subsidiaries is a “holding company,” or an “affiliate” of a “holding company,” as such terms are defined in the Public Utility Holding Act of 2005.
(tt) Federal Power Act. None of the Company nor any of its Subsidiaries is subject to regulation as a “public utility” under the Federal Power Act, as amended.
(uu) Ranking of Notes. No Indebtedness of the Company, at such Closing, will be senior to, or pari passu with, the Notes in right of payment, whether with respect to payment or redemptions, interest, damages, upon liquidation or dissolution or otherwise.
(vv) Cybersecurity. The Company and its Subsidiaries’ information technology assets and equipment, computers, systems, networks, hardware, software, websites, applications, and databases (collectively, “IT Systems”) are adequate for, and operate and perform in all material respects as required in connection with the operation of the business of the Company and its subsidiaries as currently conducted, free and clear of all material bugs, errors, defects, Trojan horses, time bombs, malware and other corruptants that would reasonably be expected to have a Material Adverse Effect on the Company’s business. The Company and its Subsidiaries have implemented and maintained commercially reasonable physical, technical and administrative controls, policies, procedures, and safeguards to maintain and protect their material confidential information and the integrity, continuous operation, redundancy and security of all IT Systems and data, including “Personal Data,” used in connection with their businesses. “Personal Data” means (i) a natural person’s name, street address, telephone number, e-mail address, photograph, social security number or tax identification number, driver’s license number, passport number, credit card number, bank information, or customer or account number; (ii) any information which would qualify as “personally identifying information” under the Federal Trade Commission Act, as amended; (iii) “personal data” as defined by the European Union General Data Protection Regulation (“GDPR”) (EU 2016/679); (iv) any information which would qualify as “protected health information” under the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act (collectively, “HIPAA”); and (v) any other piece of information that allows the identification of such natural person, or his or her family, or permits the collection or analysis of any data related to an identified person’s health or sexual orientation. There have been no breaches, violations, outages or unauthorized uses of or accesses to same, except for those that have been remedied without material cost or liability or the duty to notify any other person or such, nor any incidents under internal review or investigations relating to the same except in each case, where such would not, either individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect. The Company and its Subsidiaries are presently in compliance with all applicable laws or statutes and all judgments, orders, rules and regulations of any court or arbitrator or governmental or regulatory authority, internal policies and contractual obligations relating to the privacy and security of IT Systems and Personal Data and to the protection of such IT Systems and Personal Data from unauthorized use, access, misappropriation or modification except in each case, where such would not, either individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect.
(ww) Compliance with Data Privacy Laws. The Company and its Subsidiaries are, and at all prior times were, in compliance with all applicable state and federal data privacy and security laws and regulations, including without limitation HIPAA, and the Company and its Subsidiaries have taken commercially reasonable actions to prepare to comply with, and since May 25, 2018, have been and currently are in compliance with, the GDPR (EU 2016/679) (collectively, the “Privacy Laws”) except in each case, where such would not, either individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect. To ensure compliance with the Privacy Laws, the Company and its Subsidiaries have in place, comply with, and take appropriate steps reasonably designed to ensure compliance in all material respects with their policies and procedures relating to data privacy and security and the collection, storage, use, disclosure, handling, and analysis of Personal Data (the “Policies”). Except in each case, where such would not, either individually or in the aggregate, reasonably be expected to result in a Material Adverse Effect, the Company and its Subsidiaries have made all disclosures to users or customers required by applicable laws and regulatory rules or requirements, and none of such disclosures made or contained in any Policy have, to the
Annex B-19
knowledge of the Company, been inaccurate or in violation of any applicable laws and regulatory rules or requirements in any material respect. The Company further certifies that neither it nor any Subsidiary: (i) has received notice of any actual or potential liability under or relating to, or actual or potential violation of, any of the Privacy Laws, and has no knowledge of any event or condition that would reasonably be expected to result in any such notice; (ii) is currently conducting or paying for, in whole or in part, any investigation, remediation, or other corrective action pursuant to any Privacy Law; or (iii) is a party to any order, decree, or agreement that imposes any obligation or liability under any Privacy Law.
(xx) Disclosure. The Company confirms that neither it nor any other Person acting on its behalf has provided any of the Buyers or their agents or counsel with any information that constitutes or could reasonably be expected to constitute material, non-public information concerning the Company or any of its Subsidiaries, other than the existence of the transactions contemplated by this Agreement and the other Transaction Documents. The Company understands and confirms that each of the Buyers will rely on the foregoing representations in effecting transactions in securities of the Company. All disclosure provided to the Buyers regarding the Company and its Subsidiaries, their businesses and the transactions contemplated hereby, including the schedules to this Agreement, furnished by or on behalf of the Company or any of its Subsidiaries is true and correct and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. All of the written information furnished after the date hereof by or on behalf of the Company or any of its Subsidiaries to each Buyer pursuant to or in connection with this Agreement and the other Transaction Documents, taken as a whole, will be true and correct in all material respects as of the date on which such information is so provided and will not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. Each press release issued by the Company or any of its Subsidiaries during the twelve (12) months preceding the date of this Agreement did not at the time of release contain any untrue statement of a material fact or omit to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they are made, not misleading. No event or circumstance has occurred or information exists with respect to the Company or any of its Subsidiaries or its or their business, properties, liabilities, prospects, operations (including results thereof) or conditions (financial or otherwise), which, under applicable law, rule or regulation, requires public disclosure at or before the date hereof or announcement by the Company but which has not been so publicly disclosed. All financial projections and forecasts that have been prepared by or on behalf of the Company or any of its Subsidiaries and made available to you have been prepared in good faith based upon reasonable assumptions and represented, at the time each such financial projection or forecast was delivered to each Buyer, the Company’s best estimate of future financial performance (it being recognized that such financial projections or forecasts are not to be viewed as facts and that the actual results during the period or periods covered by any such financial projections or forecasts may differ from the projected or forecasted results). The Company acknowledges and agrees that no Buyer makes or has made any representations or warranties with respect to the transactions contemplated hereby other than those specifically set forth in Section 2.
(yy) Insurance. The Company and each of its Subsidiaries are insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as management of the Company believes to be prudent and customary in the businesses in which the Company and its Subsidiaries are engaged (including, without limitation $10,000,000 of director’s and officer’s insurance). Neither the Company nor any such Subsidiary has been refused any insurance coverage sought or applied for, and neither the Company nor any such Subsidiary has any reason to believe that it will be unable to renew its existing insurance coverage as and when such coverage expires or to obtain similar coverage from similar insurers as may be necessary to continue its business at a cost that would not have a Material Adverse Effect.
4. COVENANTS.
(a) Best Efforts. Each Buyer shall use its best efforts to timely satisfy each of the covenants hereunder and conditions to be satisfied by it as provided in Section 6 of this Agreement. The Company shall use its best efforts to timely satisfy each of the covenants hereunder and conditions to be satisfied by it as provided in Section 7 of this Agreement.
(b) Form D and Blue Sky. The Company shall file a Form D with respect to the Securities as required under Regulation D and to provide a copy thereof to each Buyer promptly after such filing. The Company shall, on or before each Closing Date, take such action as the Company shall reasonably determine is necessary in order to obtain an
Annex B-20
exemption for, or to, qualify the Securities for sale to the Buyers at the applicable Closing pursuant to this Agreement under applicable securities or “Blue Sky” laws of the states of the United States (or to obtain an exemption from such qualification), and shall provide evidence of any such action so taken to the Buyers on or prior to each Closing Date. Without limiting any other obligation of the Company under this Agreement, the Company shall timely make all filings and reports relating to the offer and sale of the Securities required under all applicable securities laws (including, without limitation, all applicable federal securities laws and all applicable “Blue Sky” laws), and the Company shall comply with all applicable foreign, federal, state and local laws, statutes, rules, regulations and the like relating to the offering and sale of the Securities to the Buyers.
(c) Reporting Status. Until the date on which the Buyers shall have sold all of the Registrable Securities (the “Reporting Period”), the Company shall timely file all reports required to be filed with the SEC pursuant to the 1934 Act, and the Company shall not terminate its status as an issuer required to file reports under the 1934 Act even if the 1934 Act or the rules and regulations thereunder would no longer require or otherwise permit such termination. From the time Form S-3 is available to the Company for the registration of the Registrable Securities, the Company shall take all actions necessary to maintain its eligibility to register the Registrable Securities for resale by the Buyers on Form S-3.
(d) Use of Proceeds. The Company will use the proceeds from the sale of the Securities for general corporate purposes, but not, directly or indirectly, for (i) except as set forth on Schedule 4(d), the satisfaction of any indebtedness of the Company or any of its Subsidiaries, (ii) the redemption or repurchase of any securities of the Company or any of its Subsidiaries, or (iii) the settlement of any outstanding litigation.
(e) Financial Information. The Company agrees to send the following to each Investor (as defined in the Registration Rights Agreement) during the Reporting Period (i) unless the following are filed with the SEC through EDGAR and are available to the public through the EDGAR system, within one (1) Business Day after the filing thereof with the SEC, a copy of its Annual Reports on Form 10-K and Quarterly Reports on Form 10-Q, any interim reports or any consolidated balance sheets, income statements, stockholders’ equity statements and/or cash flow statements for any period other than annual, any Current Reports on Form 8-K and any registration statements (other than on Form S-8) or amendments filed pursuant to the 1933 Act, (ii) unless the following are either filed with the SEC through EDGAR or are otherwise widely disseminated via a recognized news release service (such as PR Newswire), on the same day as the release thereof, e-mail copies of all press releases issued by the Company or any of its Subsidiaries and (iii) unless the following are filed with the SEC through EDGAR, copies of any notices and other information made available or given to the stockholders of the Company generally, contemporaneously with the making available or giving thereof to the stockholders.
(f) Listing. The Company shall promptly secure the listing or designation for quotation (as the case may be) of all of the Registrable Securities upon each national securities exchange and automated quotation system, if any, upon which the Common Stock is then listed or designated for quotation (as the case may be) (subject to official notice of issuance) and shall maintain such listing or designation for quotation (as the case may be) of all Registrable Securities from time to time issuable under the terms of the Transaction Documents on such national securities exchange or automated quotation system. The Company shall maintain the Common Stock’s listing or authorization for quotation (as the case may be) on The New York Stock Exchange, the NYSE American, the Nasdaq Capital Market, the Nasdaq Global Market or the Nasdaq Global Select Market (each, an “Eligible Market”). Neither the Company nor any of its Subsidiaries shall take any action which could be reasonably expected to result in the delisting or suspension of the Common Stock on an Eligible Market. The Company shall pay all fees and expenses in connection with satisfying its obligations under this Section 4(f).
(g) Fees. The Company shall reimburse the lead Buyer for all costs and expenses incurred by it or its affiliates in connection with the structuring, documentation, negotiation and closing of the transactions contemplated by the Transaction Documents (including, without limitation, as applicable, (x) at the Initial Closing, a non-accountable amount of $125,000 for the fees and expenses of Kelley Drye & Warren LLP, and (y) at each Additional Closing, a non-accountable amount of $35,000 for the fees and expenses of Kelley Drye & Warren LLP for the costs and expenses incurred by it or its affiliates in connection with the structuring, documentation, negotiation and closing of the transactions contemplated by the Transaction Documents (including, without limitation, as applicable, all legal fees of outside counsel and disbursements of Kelley Drye & Warren LLP, any other reasonable fees and expenses in connection with the structuring, documentation, negotiation and closing of the transactions contemplated by the Transaction Documents and due diligence and regulatory filings in connection therewith) (the “Transaction
Annex B-21
Expenses”)) and shall be withheld by the lead Buyer from its applicable Purchase Price at the applicable Closing, less $50,000 previously paid by the Company to any such Buyer; provided, that the Company shall promptly reimburse Kelley Drye & Warren LLP on demand for all Transaction Expenses not so reimbursed through such withholding at such Closing. The Company shall be responsible for the payment of any placement agent’s fees, financial advisory fees, Controlled Account Bank fees, transfer agent fees, DTC (as defined below) fees or broker’s commissions (other than for Persons engaged by any Buyer) relating to or arising out of the transactions contemplated hereby (including, without limitation, any fees or commissions payable to the Placement Agent, who is the Company’s sole placement agent in connection with the transactions contemplated by this Agreement). The Company shall pay, and hold each Buyer harmless against, any liability, loss or expense (including, without limitation, reasonable attorneys’ fees and out-of-pocket expenses) arising in connection with any claim relating to any such payment. Except as otherwise set forth in the Transaction Documents, each party to this Agreement shall bear its own expenses in connection with the sale of the Securities to the Buyers.
(h) Pledge of Securities. Notwithstanding anything to the contrary contained in this Agreement, the Company acknowledges and agrees that the Securities may be pledged by an Investor in connection with a bona fide margin agreement or other loan or financing arrangement that is secured by the Securities. The pledge of Securities shall not be deemed to be a transfer, sale or assignment of the Securities hereunder, and no Investor effecting a pledge of Securities shall be required to provide the Company with any notice thereof or otherwise make any delivery to the Company pursuant to this Agreement or any other Transaction Document, including, without limitation, Section 2(g) hereof; provided that an Investor and its pledgee shall be required to comply with the provisions of Section 2(g) hereof in order to effect a sale, transfer or assignment of Securities to such pledgee. The Company hereby agrees to execute and deliver such documentation as a pledgee of the Securities may reasonably request in connection with a pledge of the Securities to such pledgee by a Buyer.
(i) Disclosure of Transactions and Other Material Information.
(i) Disclosure of Transaction.
(1) Initial Closing. The Company shall, on or before 9:30 a.m., New York time, on the second (2nd) Business Day after the date of this Agreement, issue a press release (the “Initial Press Release”) reasonably acceptable to the Buyers disclosing all the material terms of the transactions contemplated by the Transaction Documents. On or before 9:30 a.m., New York time, on the second (2nd) Business Day after the date of this Agreement, the Company shall file a Current Report on Form 8-K describing all the material terms of the transactions contemplated by the Transaction Documents in the form required by the 1934 Act and attaching all the material Transaction Documents (including, without limitation, this Agreement (and all schedules to this Agreement), the form of Notes, the form of Lock-Up Agreements and the form of the Registration Rights Agreement) (the “Initial 8-K Filing”). From and after the filing of the Initial 8-K Filing, the Company shall have disclosed all material, non-public information (if any) provided to any of the Buyers by the Company or any of its Subsidiaries or any of their respective officers, directors, employees or agents in connection with the transactions contemplated by the Transaction Documents. In addition, effective upon the filing of the Initial 8-K Filing, the Company acknowledges and agrees that any and all confidentiality or similar obligations under any agreement, whether written or oral, between the Company, any of its Subsidiaries or any of their respective officers, directors, affiliates, employees or agents, on the one hand, and any of the Buyers or any of their affiliates, on the other hand, shall terminate.
(2) Additional Closings. The Company shall, on or before 9:30 a.m., New York time, on the second (2nd) Business Day after either (x) the Company receives (or delivers with respect to such applicable Additional Mandatory Closing or in its capacity of an Initiating Party to any Responding Party that elects to participate in such applicable Additional Mutual Closing) or (y) receives from a Buyer, as applicable, an Additional Closing Notice, either issue a press release (each, an “Additional Press Release”, and together with the Initial Press Release, the “Press Releases”) or file a Current Report on Form 8-K (each, an “Additional 8-K Filing”, and together with the Initial 8-K Filing, the “8-K Filings”), in each case reasonably acceptable to such Buyer participating in such Additional Closing, disclosing that, as applicable, either (I) “an institutional investor” has elected to deliver an Additional Closing Notice to the Company or (II) the Company and “an institutional investor” has mutually agreed to effect an Additional Closing. From and after the filing of the Additional Press
Annex B-22
Release or Additional 8-K Filing, solely to the extent such Additional Closing Notice constitutes material non-public information (as specified by the Company in such applicable Additional Closing Notice), the Company shall have disclosed all material, non-public information (if any) provided to any of the Buyers by the Company or any of its Subsidiaries or any of their respective officers, directors, employees or agents in connection with the transactions contemplated by the Transaction Documents. In addition, effective upon the filing of the Additional 8-K Filing, the Company acknowledges and agrees that any and all confidentiality or similar obligations under any agreement, whether written or oral, between the Company, any of its Subsidiaries or any of their respective officers, directors, affiliates, employees or agents, on the one hand, and any of the Buyers or any of their affiliates, on the other hand, shall terminate.
(ii) Limitations on Disclosure. The Company shall not, and the Company shall cause each of its Subsidiaries and each of its and their respective officers, directors, employees and agents not to, provide any Buyer with any material, non-public information regarding the Company or any of its Subsidiaries from and after the date hereof without the express prior written consent of such Buyer (which may be granted or withheld in such Buyer’s sole discretion). In the event of a breach of any of the foregoing covenants, including, without limitation, Section 4(o) of this Agreement, or any of the covenants or agreements contained in any other Transaction Document, by the Company, any of its Subsidiaries, or any of its or their respective officers, directors, employees and agents (as determined in the reasonable good faith judgment of such Buyer), in addition to any other remedy provided herein or in the Transaction Documents, such Buyer shall have the right to make a public disclosure, in the form of a press release, public advertisement or otherwise, of such breach or such material, non-public information, as applicable, without the prior approval by the Company, any of its Subsidiaries, or any of its or their respective officers, directors, employees or agents. No Buyer shall have any liability to the Company, any of its Subsidiaries, or any of its or their respective officers, directors, employees, affiliates, stockholders or agents, for any such disclosure. To the extent that the Company delivers any material, non-public information to a Buyer without such Buyer’s consent, the Company hereby covenants and agrees that such Buyer shall not have any duty of confidentiality with respect to, or a duty not to trade on the basis of, such material, non-public information. Subject to the foregoing, neither the Company, its Subsidiaries nor any Buyer shall issue any press releases or any other public statements with respect to the transactions contemplated hereby; provided, however, the Company shall be entitled, without the prior approval of any Buyer, to make the Press Releases and any press release or other public disclosure with respect to such transactions (i) in substantial conformity with the 8-K Filings and contemporaneously therewith and (ii) as is required by applicable law and regulations (provided that in the case of clause (i) each Buyer shall be consulted by the Company in connection with any such press release or other public disclosure prior to its release). Without the prior written consent of the applicable Buyer (which may be granted or withheld in such Buyer’s sole discretion), the Company shall not (and shall cause each of its Subsidiaries and affiliates to not) disclose the name of such Buyer in any filing, announcement, release or otherwise. Notwithstanding anything contained in this Agreement to the contrary and without implication that the contrary would otherwise be true, the Company expressly acknowledges and agrees that no Buyer shall have (unless expressly agreed to by a particular Buyer after the date hereof in a written definitive and binding agreement executed by the Company and such particular Buyer (it being understood and agreed that no Buyer may bind any other Buyer with respect thereto)), any duty of confidentiality with respect to, or a duty not to trade on the basis of, any material, non-public information regarding the Company or any of its Subsidiaries.
(iii) Other Confidential Information. Disclosure Failures; Disclosure Delay Payments. In addition to other remedies set forth in this Section 4(i), and without limiting anything set forth in any other Transaction Document, at any time after each Closing Date if the Company, any of its Subsidiaries, or any of their respective officers, directors, employees or agents, provides any Buyer with material non-public information relating to the Company or any of its Subsidiaries (each, the “Confidential Information”), the Company shall, on or prior to the applicable Required Disclosure Date (as defined below), publicly disclose such Confidential Information on a Current Report on Form 8-K or otherwise (each, a “Disclosure”). From and after such Disclosure, the Company shall have disclosed all Confidential Information provided to such Buyer by the Company or any of its Subsidiaries or any of their respective officers, directors, employees or agents in connection with the transactions contemplated by the Transaction Documents. In addition, effective upon such Disclosure, the Company acknowledges and agrees that any and all confidentiality or similar obligations under any agreement, whether written or oral, between the Company, any of its Subsidiaries or any of their
Annex B-23
respective officers, directors, affiliates, employees or agents, on the one hand, and any of the Buyers or any of their affiliates, on the other hand, shall terminate. In the event that the Company fails to effect such Disclosure on or prior to the Required Disclosure Date and such Buyer shall have possessed Confidential Information for at least ten (10) consecutive Trading Days (each, a “Disclosure Failure”), then, as partial relief for the damages to such Buyer by reason of any such delay in, or reduction of, its ability to buy or sell shares of Common Stock after such Required Disclosure Date (which remedy shall not be exclusive of any other remedies available at law or in equity), the Company shall pay to such Buyer an amount in cash equal to the greater of (I) one percent (1%) of the aggregate Purchase Price and (II) the applicable Disclosure Restitution Amount, on each of the following dates (each, a “Disclosure Delay Payment Date”): (i) on the date of such Disclosure Failure and (ii) on every thirty (30) day anniversary such Disclosure Failure until the earlier of (x) the date such Disclosure Failure is cured and (y) such time as all such non-public information provided to such Buyer shall cease to be Confidential Information (as evidenced by a certificate, duly executed by an authorized officer of the Company to the foregoing effect) (such earlier date, as applicable, a “Disclosure Cure Date”). Following the initial Disclosure Delay Payment for any particular Disclosure Failure, without limiting the foregoing, if a Disclosure Cure Date occurs prior to any thirty (30) day anniversary of such Disclosure Failure, then such Disclosure Delay Payment (prorated for such partial month) shall be made on the second (2nd) Business Day after such Disclosure Cure Date. The payments to which an Investor shall be entitled pursuant to this Section 4(l)(iii) are referred to herein as “Disclosure Delay Payments.” In the event the Company fails to make Disclosure Delay Payments in a timely manner in accordance with the foregoing, such Disclosure Delay Payments shall bear interest at the rate of one percent (1%) per month (prorated for partial months) until paid in full.
(iv) For the purpose of this Agreement the following definitions shall apply:
(1) “Disclosure Failure Market Price” means, as of any Disclosure Delay Payment Date, the price computed as the quotient of (I) the sum of the five (5) highest VWAPs (as defined in the Notes) of the Common Stock during the applicable Disclosure Restitution Period (as defined below), divided by (II) five (5) (such period, the “Disclosure Failure Measuring Period”). All such determinations to be appropriately adjusted for any share dividend, share split, share combination, reclassification or similar transaction that proportionately decreases or increases the Common Stock during such Disclosure Failure Measuring Period.
(2) “Disclosure Restitution Amount” means, as of any Disclosure Delay Payment Date, the product of (x) difference of (I) the Disclosure Failure Market Price less (II) the lowest purchase price, per share of Common Stock, of any Common Stock issued or issuable to such Buyer pursuant to this Agreement or any other Transaction Documents, multiplied by (y) 10% of the aggregate daily dollar trading volume (as reported on Bloomberg (as defined in the Notes)) of the Common Stock on the Principal Market for each Trading Day (as defined in the Notes) either (1) with respect to the initial Disclosure Delay Payment Date, during the period commencing on the applicable Required Disclosure Date through and including the Trading Day immediately prior to the initial Disclosure Delay Payment Date or (2) with respect to each other Disclosure Delay Payment Date, during the period commencing the immediately preceding Disclosure Delay Payment Date through and including the Trading Day immediately prior to such applicable Disclosure Delay Payment Date (such applicable period, the “Disclosure Restitution Period”).
(3) “Required Disclosure Date” means (x) if such Buyer authorized the delivery of such Confidential Information, either (I) if the Company and such Buyer have mutually agreed upon a date (as evidenced by an e-mail or other writing) of Disclosure of such Confidential Information, such agreed upon date or (II) otherwise, the seventh (7th) calendar day after the date such Buyer first received any Confidential Information or (y) if such Buyer did not authorize the delivery of such Confidential Information, the first (1st) Business Day after such Buyer’s receipt of such Confidential Information.
(j) Additional Registration Statements. Until the Applicable Date (as defined below) and at any time thereafter while any Registration Statement is not effective or the prospectus contained therein is not available for use or any Current Public Information Failure (as defined in the Registration Rights Agreement) exists, the Company shall not file a registration statement or an offering statement under the 1933 Act relating to securities that are not
Annex B-24
the Registrable Securities (other than a registration statement on Form S-8 or such supplements or amendments to registration statements that are outstanding and have been declared effective by the SEC as of the date hereof (solely to the extent necessary to keep such registration statements effective and available and not with respect to any Subsequent Placement)). “Applicable Date” means, with respect to any given Closing, the later of (i) the applicable Closing Date and (ii) the earlier of (x) the first date on which the resale by the Buyers of all the Registrable Securities required to be filed on the initial Registration Statement with respect to such Closing pursuant to the Registration Rights Agreement is declared effective by the SEC (and each prospectus contained therein is available for use on such date) or (y) the first date on which all of the Registrable Securities with respect to the Securities issued at such Closing are eligible to be resold by the Buyers pursuant to Rule 144 (or, if a Current Public Information Failure has occurred and is continuing, such later date after which the Company has cured such Current Public Information Failure).
(k) Additional Issuance of Securities. Until the later of (x) the Additional Closing Expiration Date and (y) such date no Notes remain outstanding (such later date, the “Covenant Defeasance Date”), the Company will not, without the prior written consent of the Required Holders, issue any Notes (other than to the Buyers as contemplated hereby) and the Company shall not issue any other securities that would cause a breach or default under the Notes. The Company agrees that for the period commencing (i) with respect to the Initial Closing, on the date hereof or, with respect to any Additional Closing, on the Additional Closing Notice Date of such Additional Closing and ending on the date immediately following the 60h Trading Day after the Applicable Date of such Closing; provided that such period shall be extended by the number of calendar days during such period and any extension thereof contemplated by this proviso on which any Registration Statement is not effective or any prospectus contained therein is not available for use or any Current Public Information Failure exists (each, a “Restricted Period”), neither the Company nor any of its Subsidiaries shall directly or indirectly issue, offer, sell, grant any option or right to purchase, or otherwise dispose of (or announce any issuance, offer, sale, grant of any option or right to purchase or other disposition of) any equity security or any equity-linked or related security (including, without limitation, any “equity security” (as that term is defined under Rule 405 promulgated under the 1933 Act), any Common Stock Equivalents (as defined below), any debt, any preferred stock or any purchase rights) (any such issuance, offer, sale, grant, disposition or announcement (whether occurring during the Restricted Period or at any time thereafter) is referred to as a “Subsequent Placement”). Notwithstanding the foregoing, this Section 4(k) shall not apply in respect of the issuance of (i) shares of Common Stock or standard options to purchase Common Stock to directors, officers or employees of the Company in their capacity as such pursuant to an Approved Stock Plan (as defined below), provided that (1) all such issuances (taking into account the shares of Common Stock issuable upon exercise of such options) after the date hereof pursuant to this clause (i) do not, in the aggregate, exceed more than 7% of the Common Stock issued and outstanding immediately prior to the date hereof and (2) the exercise price of any such options is not lowered, none of such options are amended to increase the number of shares issuable thereunder and none of the terms or conditions of any such options are otherwise materially changed in any manner that adversely affects any of the Buyers; (ii) shares of Common Stock issued upon the conversion or exercise of Common Stock Equivalents (other than standard options to purchase Common Stock issued pursuant to an Approved Stock Plan that are covered by clause (i) above) issued prior to the date hereof, provided that the conversion, exercise or other method of issuance (as the case may be) of any such Common Stock Equivalent is made solely pursuant to the conversion, exercise or other method of issuance (as the case may be) provisions of such Common Stock Equivalent that were in effect on the date immediately prior to the date of this Agreement, the conversion, exercise or issuance price of any such Common Stock Equivalents (other than standard options to purchase Common Stock issued pursuant to an Approved Stock Plan that are covered by clause (i) above) is not lowered, none of such Common Stock Equivalents (other than standard options to purchase Common Stock issued pursuant to an Approved Stock Plan that are covered by clause (i) above) are amended to increase the number of shares issuable thereunder and none of the terms or conditions of any such Common Stock Equivalents (other than standard options to purchase Common Stock issued pursuant to an Approved Stock Plan that are covered by clause (i) above) are otherwise materially changed in any manner that adversely affects any of the Buyers; (iii) the Conversion Shares and (iv) the securities to be issued in the Transactions in accordance with the terms of the Merger Agreement, as in effect as of the date hereof, (each of the foregoing in clauses (i) through (iv), collectively the “Excluded Securities”). “Approved Stock Plan” means any employee benefit plan which has been approved by the board of directors of the Company prior to or subsequent to the date hereof pursuant to which shares of Common Stock and standard options to purchase Common Stock may be issued to any employee, officer or director for services provided to the Company in their capacity as such.
Annex B-25
(l) Reservation of Shares. Until the Covenant Defeasance Date, the Company shall take all action necessary to, at all times, have authorized, and reserved for the purpose of issuance, no less than 300% of the maximum number of Conversion Shares issuable upon conversion of the Notes then outstanding (assuming for purposes hereof that (w) the Initial Closing has occurred and all Notes issuable at Additional Optional Closings have been issued hereunder, and (x) any such conversion shall not take into account any limitations on the conversion of the Notes set forth in the Notes)(collectively, the “Required Reserve Amount”); provided that at no time shall the number of shares of Common Stock reserved pursuant to this Section 4(l) be reduced other than proportionally in connection with any conversion and/or redemption, as applicable, of Notes. If at any time the number of shares of Common Stock authorized and reserved for issuance is not sufficient to meet the Required Reserve Amount, the Company will promptly take all corporate action necessary to authorize and reserve a sufficient number of shares, including, without limitation, calling a special meeting of stockholders to authorize additional shares to meet the Company’s obligations pursuant to the Transaction Documents, in the case of an insufficient number of authorized shares, obtain stockholder approval of an increase in such authorized number of shares, and voting the management shares of the Company in favor of an increase in the authorized shares of the Company to ensure that the number of authorized shares is sufficient to meet the Required Reserve Amount.
(m) Conduct of Business. The business of the Company and its Subsidiaries shall not be conducted in violation of any law, ordinance or regulation of any Governmental Entity, except where such violations would not reasonably be expected to result, either individually or in the aggregate, in a Material Adverse Effect.
(n) Other Notes; Variable Securities. Until the Covenant Defeasance Date, the Company and each Subsidiary shall be prohibited from effecting or entering into an agreement to effect any Subsequent Placement involving a Variable Rate Transaction. “Variable Rate Transaction” means a transaction in which the Company or any Subsidiary (i) issues or sells any Common Stock Equivalents either (A) at a conversion, exercise or exchange rate or other price that is based upon and/or varies with the trading prices of or quotations for the shares of Common Stock at any time after the initial issuance of such Common Stock Equivalents, or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial issuance of such Common Stock Equivalents or upon the occurrence of specified or contingent events directly or indirectly related to the business of the Company or the market for the Common Stock, other than pursuant to a customary “weighted average” anti-dilution provision or (ii) enters into any agreement (including, without limitation, an equity line of credit or an “at-the-market” offering) whereby the Company or any Subsidiary may sell securities at a future determined price (other than standard and customary “preemptive” or “participation” rights). Each Buyer shall be entitled to obtain injunctive relief against the Company and its Subsidiaries to preclude any such issuance, which remedy shall be in addition to any right to collect damages.
(o) Participation Right. At any time on or prior to the six month anniversary of the Additional Closing Expiration Date (or, if later, the date no Notes remain outstanding), neither the Company nor any of its Subsidiaries shall, directly or indirectly, effect any Subsequent Placement unless the Company shall have first complied with this Section 4(o). The Company acknowledges and agrees that the right set forth in this Section 4(o) is a right granted by the Company, separately, to each Buyer.
(i) At least five (5) Trading Days prior to any proposed or intended Subsequent Placement, the Company shall deliver to each Buyer a written notice (each such notice, a “Pre-Notice”), which Pre-Notice shall not contain any information (including, without limitation, material, non-public information) other than: (A) if the proposed Offer Notice (as defined below) constitutes or contains material, non-public information, a statement asking whether the Investor is willing to accept material non-public information or (B) if the proposed Offer Notice does not constitute or contain material, non-public information, (x) a statement that the Company proposes or intends to effect a Subsequent Placement, (y) a statement that the statement in clause (x) above does not constitute material, non-public information and (z) a statement informing such Buyer that it is entitled to receive an Offer Notice (as defined below) with respect to such Subsequent Placement upon its written request. Upon the written request of a Buyer within three (3) Trading Days after the Company’s delivery to such Buyer of such Pre-Notice, and only upon a written request by such Buyer, the Company shall promptly, but no later than one (1) Trading Day after such request, deliver to such Buyer an irrevocable written notice (the “Offer Notice”) of any proposed or intended issuance or sale or exchange (the “Offer”) of the securities being offered (the “Offered Securities”) in a Subsequent Placement, which Offer Notice shall (A) identify and describe the Offered Securities, (B) describe the price and other terms upon which they are to be issued, sold or exchanged, and the number or amount of the Offered Securities to be issued, sold or exchanged, (C) identify the Persons (if known) to which or with which the Offered Securities are to be offered,
Annex B-26
issued, sold or exchanged and (D) offer to issue and sell to or exchange with such Buyer in accordance with the terms of the Offer 30% of the Offered Securities, provided that the number of Offered Securities which such Buyer shall have the right to subscribe for under this Section 4(o) shall be (x) based on such Buyer’s pro rata portion of the aggregate original principal amount of the Notes purchased hereunder by all Buyers (the “Basic Amount”), and (y) with respect to each Buyer that elects to purchase its Basic Amount, any additional portion of the Offered Securities attributable to the Basic Amounts of other Buyers as such Buyer shall indicate it will purchase or acquire should the other Buyers subscribe for less than their Basic Amounts (the “Undersubscription Amount”), which process shall be repeated until each Buyer shall have an opportunity to subscribe for any remaining Undersubscription Amount.
(ii) To accept an Offer, in whole or in part, such Buyer must deliver a written notice to the Company prior to the end of the fifth (5th) Business Day after such Buyer’s receipt of the Offer Notice (the “Offer Period”), setting forth the portion of such Buyer’s Basic Amount that such Buyer elects to purchase and, if such Buyer shall elect to purchase all of its Basic Amount, the Undersubscription Amount, if any, that such Buyer elects to purchase (in either case, the “Notice of Acceptance”). If the Basic Amounts subscribed for by all Buyers are less than the total of all of the Basic Amounts, then each Buyer who has set forth an Undersubscription Amount in its Notice of Acceptance shall be entitled to purchase, in addition to the Basic Amounts subscribed for, the Undersubscription Amount it has subscribed for; provided, however, if the Undersubscription Amounts subscribed for exceed the difference between the total of all the Basic Amounts and the Basic Amounts subscribed for (the “Available Undersubscription Amount”), each Buyer who has subscribed for any Undersubscription Amount shall be entitled to purchase only that portion of the Available Undersubscription Amount as the Basic Amount of such Buyer bears to the total Basic Amounts of all Buyers that have subscribed for Undersubscription Amounts, subject to rounding by the Company to the extent it deems reasonably necessary. Notwithstanding the foregoing, if the Company desires to modify or amend the terms and conditions of the Offer prior to the expiration of the Offer Period, the Company may deliver to each Buyer a new Offer Notice and the Offer Period shall expire on the fifth (5th) Business Day after such Buyer’s receipt of such new Offer Notice.
(iii) The Company shall have five (5) Business Days from the expiration of the Offer Period above (A) to offer, issue, sell or exchange all or any part of such Offered Securities as to which a Notice of Acceptance has not been given by a Buyer (the “Refused Securities”) pursuant to a definitive agreement(s) (the “Subsequent Placement Agreement”), but only to the offerees described in the Offer Notice (if so described therein) and only upon terms and conditions (including, without limitation, unit prices and interest rates) that are not more favorable to the acquiring Person or Persons or less favorable to the Company than those set forth in the Offer Notice and (B) to publicly announce (x) the execution of such Subsequent Placement Agreement, and (y) either (I) the consummation of the transactions contemplated by such Subsequent Placement Agreement or (II) the termination of such Subsequent Placement Agreement, which shall be filed with the SEC on a Current Report on Form 8-K with such Subsequent Placement Agreement and any documents contemplated therein filed as exhibits thereto.
(iv) In the event the Company shall propose to sell less than all the Refused Securities (any such sale to be in the manner and on the terms specified in Section 4(o)(iii) above), then each Buyer may, at its sole option and in its sole discretion, withdraw its Notice of Acceptance or reduce the number or amount of the Offered Securities specified in its Notice of Acceptance to an amount that shall be not less than the number or amount of the Offered Securities that such Buyer elected to purchase pursuant to Section 4(o)(ii) above multiplied by a fraction, (i) the numerator of which shall be the number or amount of Offered Securities the Company actually proposes to issue, sell or exchange (including Offered Securities to be issued or sold to Buyers pursuant to this Section 4(o) prior to such reduction) and (ii) the denominator of which shall be the original amount of the Offered Securities. In the event that any Buyer so elects to reduce the number or amount of Offered Securities specified in its Notice of Acceptance, the Company may not issue, sell or exchange more than the reduced number or amount of the Offered Securities unless and until such securities have again been offered to the Buyers in accordance with Section 4(o)(i) above.
(v) Upon the closing of the issuance, sale or exchange of all or less than all of the Refused Securities, such Buyer shall acquire from the Company, and the Company shall issue to such Buyer, the number or amount of Offered Securities specified in its Notice of Acceptance, as reduced pursuant to Section 4(o)(iv) above if such Buyer has so elected, upon the terms and conditions specified in the Offer. The purchase by
Annex B-27
such Buyer of any Offered Securities is subject in all cases to the preparation, execution and delivery by the Company and such Buyer of a separate purchase agreement relating to such Offered Securities reasonably satisfactory in form and substance to such Buyer and its counsel.
(vi) Any Offered Securities not acquired by a Buyer or other Persons in accordance with this Section 4(o) may not be issued, sold or exchanged until they are again offered to such Buyer under the procedures specified in this Agreement.
(vii) The Company and each Buyer agree that if any Buyer elects to participate in the Offer, (x) neither the Subsequent Placement Agreement with respect to such Offer nor any other transaction documents related thereto (collectively, the “Subsequent Placement Documents”) shall include any term or provision whereby such Buyer shall be required to agree to any restrictions on trading as to any securities of the Company or be required to consent to any amendment to or termination of, or grant any waiver, release or the like under or in connection with, any agreement previously entered into with the Company or any instrument received from the Company, and (y) any registration rights set forth in such Subsequent Placement Documents shall be similar in all material respects to the registration rights contained in the Registration Rights Agreement.
(viii) Notwithstanding anything to the contrary in this Section 4(o) and unless otherwise agreed to by such Buyer, the Company shall either confirm in writing to such Buyer that the transaction with respect to the Subsequent Placement has been abandoned or shall publicly disclose its intention to issue the Offered Securities, in either case, in such a manner such that such Buyer will not be in possession of any material, non-public information, by the fifth (5th) Business Day following delivery of the Offer Notice. If by such fifth (5th) Business Day, no public disclosure regarding a transaction with respect to the Offered Securities has been made, and no notice regarding the abandonment of such transaction has been received by such Buyer, such transaction shall be deemed to have been abandoned and such Buyer shall not be in possession of any material, non-public information with respect to the Company or any of its Subsidiaries. Should the Company decide to pursue such transaction with respect to the Offered Securities, the Company shall provide such Buyer with another Offer Notice and such Buyer will again have the right of participation set forth in this Section 4(o). The Company shall not be permitted to deliver more than one such Offer Notice to such Buyer in any sixty (60) day period, except as expressly contemplated by the last sentence of Section 4(o)(ii).
(ix) The restrictions contained in this Section 4(o) shall not apply in connection with the issuance of any Excluded Securities. The Company shall not circumvent the provisions of this Section 4(o) by providing terms or conditions to one Buyer that are not provided to all.
(p) Passive Foreign Investment Company. Until the Covenant Defeasance Date, the Company shall conduct its business, and shall cause its Subsidiaries to conduct their respective businesses, in such a manner as will ensure that the Company will not be deemed to constitute a passive foreign investment company within the meaning of Section 1297 of the Code.
(q) Restriction on Redemption and Cash Dividends. Until the Covenant Defeasance Date, the Company shall not, directly or indirectly, redeem, or declare or pay any cash dividend or distribution on, any securities of the Company without the prior express written consent of the Buyers.
(r) Corporate Existence. Until the Covenant Defeasance Date, the Company shall not be party to any Fundamental Transaction (as defined in the Notes) unless the Company is in compliance with the applicable provisions governing Fundamental Transactions set forth in the Notes.
(s) Stock Splits. Except as set forth in the Merger Agreement, until the Covenant Defeasance Date, the Company shall not effect any stock combination, reverse stock split or other similar transaction (or make any public announcement or disclosure with respect to any of the foregoing) without the prior written consent of the Required Holders (as defined below).
(t) Conversion Procedures. Each of the form of Conversion Notice (as defined in the Notes) included in the Notes set forth the totality of the procedures required of the Buyers in order to convert the Notes. Except as provided in Section 5(d), no additional legal opinion, other information or instructions shall be required of the Buyers to convert their Notes. The Company shall honor conversions of the Notes and shall deliver the Conversion Shares in accordance with the terms, conditions and time periods set forth in the Notes.
Annex B-28
(u) Regulation M. The Company will not take any action prohibited by Regulation M under the 1934 Act, in connection with the distribution of the Securities contemplated hereby.
(v) General Solicitation. None of the Company, any of its affiliates (as defined in Rule 501(b) under the 1933 Act) or any person acting on behalf of the Company or such affiliate will solicit any offer to buy or offer or sell the Securities by means of any form of general solicitation or general advertising within the meaning of Regulation D, including: (i) any advertisement, article, notice or other communication published in any newspaper, magazine or similar medium or broadcast over television or radio; and (ii) any seminar or meeting whose attendees have been invited by any general solicitation or general advertising.
(w) Integration. None of the Company, any of its affiliates (as defined in Rule 501(b) under the 1933 Act), or any person acting on behalf of the Company or such affiliate will sell, offer for sale, or solicit offers to buy or otherwise negotiate in respect of any security (as defined in the 1933 Act) which will be integrated with the sale of the Securities in a manner which would require the registration of the Securities under the 1933 Act or require stockholder approval under the rules and regulations of the Principal Market and the Company will take all action that is appropriate or necessary to assure that its offerings of other securities will not be integrated for purposes of the 1933 Act or the rules and regulations of the Principal Market, with the issuance of Securities contemplated hereby.
(x) Notice of Disqualification Events. The Company will notify the Buyers in writing, prior to each Closing Date of (i) any Disqualification Event relating to any Issuer Covered Person and (ii) any event that would, with the passage of time, become a Disqualification Event relating to any Issuer Covered Person.
(y) Stockholder Consent. On or prior to the Initial Closing Date, the Company shall obtain the written consent of the requisite stockholders (the “Stockholder Consent”) to the issuance of all of the Securities in compliance with the rules and regulations of the Principal Market (without regard to any limitations on conversion set forth in the Notes) (such affirmative approval being referred to herein as the “Stockholder Approval”, and the date the information statement filed with the SEC with respect thereto is effective, the “Stockholder Approval Date”). The Company shall inform the stockholders of the Company of the receipt of the Stockholder Consent by preparing and filing with the SEC, as promptly as practicable after the date hereof, but prior to the thirtieth (30th) calendar day after the Initial Closing Date an information statement with respect thereto, which shall be effective as promptly as practicable thereafter, but prior to the one hundred and twentieth (120th) calendar day after the Initial Closing Date (the “Stockholder Approval Deadline”)
(z) No Net Short Position Buyer hereby agrees solely with the Company, severally and not jointly, and not with any other Buyer, for so long as such Buyer owns any Notes, so long as no Event of Default has occurred and is continuing, such Buyer shall not maintain a Net Short Position (as defined below). For purposes hereof, a “Net Short Position” by a person means a position whereby such person has executed one or more sales of Common Stock that is marked as a short sale (but not including any sale marked “short exempt”) and that is executed at a time when such Buyer has no equivalent offsetting long position in the Common Stock (or is deemed to have a long position hereunder or otherwise in accordance with Regulation SHO of the 1934 Act); provided, that, for purposes of such calculations, any short sales either (x) consummated at a price greater than or equal to the Conversion Price, (y) that is a result of a bona-fide trading error on behalf of such Buyer (or its affiliates) or (z) that would otherwise be marked as a “long” sale, but for the occurrence of a Conversion Failure (as defined in the Notes) or any other breach by the Company (or its affiliates or agents, including, without limitation, the Transfer Agent (as defined below)) of any Transaction Document, in each case, shall be excluded from such calculations. For purposes of determining whether a Buyer has an equivalent offsetting “long” position in the Common Stock, (A) all Common Stock that is owned by such Buyer shall be deemed held “long” by such Buyer, (B) at any time a Conversion Notice (as defined in the Notes) is delivered by such Buyer to the Company, any shares of Common Stock issued or issuable to such Buyer (or its designee, if applicable) in connection therewith shall be deemed held “long” by such Buyer from and after the date of such Conversion Notice until such time as such Buyer shall no longer beneficially own such shares of Common Stock, and (C) at any other time the Company is required (or has elected (or is deemed to have elected)) to issue shares of Common Stock to such Buyer pursuant to the terms of the Notes, any shares of Common Stock issued or issuable to such Buyer (or its designee, if applicable) in connection therewith shall be deemed held “long” by such Buyer from and after the date that is one (1) Trading Day prior to the deadline for delivery of such shares of Common Stock to such Buyer, as set forth in the Notes, until such time as such Buyer shall no longer beneficially own such shares of Common Stock.
Annex B-29
(aa) No Waiver of Voting Agreement. The Company shall not amend, waive, modify or fail to use reasonable best efforts to enforce any provision of any of the Voting Agreements. For the avoidance of doubt, no Buyer shall be a third party beneficiary of any Voting Agreement.
(bb) Return of Pre-Delivery Shares. Each Buyer, severally, hereby covenants and agrees that if such Buyer holds any Pre-Delivery Shares after such date that no Notes are then held by such Buyer (but after any applicable Delivery Share Applications (as defined in the Notes) related thereto), such remaining Pre-Delivery Shares shall be promptly returned by such Buyer to the Company for cancellation.
(cc) Holding Period. For the purposes of Rule 144, the Company acknowledges that the holding period of the Pre-Delivery Shares may be tacked onto the holding period of the Notes and shall, consequently, be deemed to have been issued as of the Closing Date for purposes of Rule 144 and the Company agrees not to take a position contrary to this Section 4(cc).
(dd) Closing Documents. On or prior to fourteen (14) calendar days after each Closing Date, the Company agrees to deliver, or cause to be delivered, to each Buyer and Kelley Drye & Warren LLP a complete closing set of the executed Transaction Documents, Securities and any other document required to be delivered to any party pursuant to Section 7 hereof or otherwise.
5. REGISTER; TRANSFER AGENT INSTRUCTIONS; LEGEND.
(a) Register. The Company shall maintain at its principal executive offices (or such other office or agency of the Company as it may designate by notice to each holder of Securities), a register for the Notes in which the Company shall record the name and address of the Person in whose name the Notes have been issued (including the name and address of each transferee), the principal amount of the Notes held by such Person and the number of Conversion Shares issuable pursuant to the terms of the Notes held by such Person. The Company shall keep the register open and available during business hours for inspection of any Buyer or its legal representatives at any time upon reasonable notice to the Company.
(b) Transfer Agent Instructions. The Company shall issue irrevocable instructions to its transfer agent and any subsequent transfer agent (as applicable, the “Transfer Agent”) in a form acceptable to each of the Buyers (the “Irrevocable Transfer Agent Instructions”) to issue certificates or credit shares to the applicable balance accounts at The Depository Trust Company (“DTC”), registered in the name of each Buyer or its respective nominee(s), for the Conversion Shares in such amounts as specified from time to time by each Buyer to the Company upon conversion of the Notes. The Company represents and warrants that no instruction other than the Irrevocable Transfer Agent Instructions referred to in this Section 5(b), and stop transfer instructions to give effect to Section 2(g) hereof, will be given by the Company to the Transfer Agent with respect to the Securities, and that the Securities shall otherwise be freely transferable on the books and records of the Company, as applicable, to the extent provided in this Agreement and the other Transaction Documents. Notwithstanding the foregoing, if at any time the Irrevocable Transfer Agent Instructions fails to irrevocably instruct the Transfer Agent to reserve at least the Required Reserve Amount of Common Stock as of such applicable time of determination, the Company shall promptly deliver an updated irrevocable transfer agent instructions to the Transfer Agent, which shall irrevocably instruct the Transfer Agent to increase such reserve with respect to the Securities to at least the Required Reserve Amount of Common Stock as of such time of determination. If a Buyer effects a sale, assignment or transfer of the Securities in accordance with Section 2(g), the Company shall permit the transfer and shall promptly instruct the Transfer Agent to issue one or more certificates or credit shares to the applicable balance accounts at DTC in such name and in such denominations as specified by such Buyer to effect such sale, transfer or assignment. In the event that such sale, assignment or transfer involves Conversion Shares sold, assigned or transferred pursuant to an effective registration statement or in compliance with Rule 144, the Transfer Agent shall issue such shares to such Buyer, assignee or transferee (as the case may be) without any restrictive legend in accordance with Section 5(d) below. The Company acknowledges that a breach by it of its obligations hereunder will cause irreparable harm to a Buyer. Accordingly, the Company acknowledges that the remedy at law for a breach of its obligations under this Section 5(b) will be inadequate and agrees, in the event of a breach or threatened breach by the Company of the provisions of this Section 5(b), that a Buyer shall be entitled, in addition to all other available remedies, to an order and/or injunction restraining any breach and requiring immediate issuance and transfer, without the necessity of showing economic loss and without any bond or other security being required. The Company shall cause its counsel to issue the legal opinion referred to in the Irrevocable Transfer Agent Instructions to the Transfer Agent on each Effective Date (as defined in the Registration Rights Agreement). Any fees (with respect to the Transfer Agent, counsel to the Company or otherwise) associated with the issuance of such opinion or the removal of any legends on any of the Securities shall be borne by the Company.
Annex B-30
(c) Legends. Each Buyer understands that the Securities have been issued (or will be issued in the case of the Conversion Shares) pursuant to an exemption from registration or qualification under the 1933 Act and applicable state securities laws, and except as set forth below, the Securities shall bear any legend as required by the “blue sky” laws of any state and a restrictive legend in substantially the following form (and a stop-transfer order may be placed against transfer of such stock certificates):
[NEITHER THE ISSUANCE AND SALE OF THE SECURITIES REPRESENTED BY THIS CERTIFICATE NOR THE SECURITIES INTO WHICH THESE SECURITIES ARE CONVERTIBLE HAVE BEEN][THE SECURITIES REPRESENTED BY THIS CERTIFICATE HAVE NOT BEEN] REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR APPLICABLE STATE SECURITIES LAWS. THE SECURITIES MAY NOT BE OFFERED FOR SALE, SOLD, TRANSFERRED OR ASSIGNED (I) IN THE ABSENCE OF (A) AN EFFECTIVE REGISTRATION STATEMENT FOR THE SECURITIES UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR (B) AN OPINION OF COUNSEL TO THE HOLDER (IF REQUESTED BY THE COMPANY), IN A FORM REASONABLY ACCEPTABLE TO THE COMPANY, THAT REGISTRATION IS NOT REQUIRED UNDER SAID ACT OR (II) UNLESS SOLD OR ELIGIBLE TO BE SOLD PURSUANT TO RULE 144 OR RULE 144A UNDER SAID ACT. NOTWITHSTANDING THE FOREGOING, THE SECURITIES MAY BE PLEDGED IN CONNECTION WITH A BONA FIDE MARGIN ACCOUNT OR OTHER LOAN OR FINANCING ARRANGEMENT SECURED BY THE SECURITIES.
(d) Removal of Legends. Certificates evidencing Securities shall not be required to contain the legend set forth in Section 5(c) above or any other legend (i) while a registration statement (including a Registration Statement) covering the resale of such Securities is effective under the 1933 Act, (ii) following any sale of such Securities pursuant to Rule 144 (assuming the transferor is not an affiliate of the Company), (iii) if such Securities are eligible to be sold, assigned or transferred under Rule 144 (provided that a Buyer provides the Company with reasonable assurances that such Securities are eligible for sale, assignment or transfer under Rule 144 which shall not include an opinion of Buyer’s counsel), (iv) in connection with a sale, assignment or other transfer (other than under Rule 144), provided that such Buyer provides the Company with an opinion of counsel to such Buyer, in a generally acceptable form, to the effect that such sale, assignment or transfer of the Securities may be made without registration under the applicable requirements of the 1933 Act or (v) if such legend is not required under applicable requirements of the 1933 Act (including, without limitation, controlling judicial interpretations and pronouncements issued by the SEC). If a legend is not required pursuant to the foregoing, the Company shall no later than two (2) Trading Days (or such earlier date as required pursuant to the 1934 Act or other applicable law, rule or regulation for the settlement of a trade initiated on the date such Buyer delivers such legended certificate representing such Securities to the Company) following the delivery by a Buyer to the Company or the Transfer Agent (with notice to the Company) of a legended certificate representing such Securities (endorsed or with stock powers attached, signatures guaranteed, and otherwise in form necessary to affect the reissuance and/or transfer, if applicable), together with any other deliveries from such Buyer as may be required above in this Section 5(d), as directed by such Buyer, either: (A) provided that the Transfer Agent is participating in the DTC Fast Automated Securities Transfer Program (“FAST”) and such Securities are Conversion Shares, credit the aggregate number of shares of Common Stock to which such Buyer shall be entitled to such Buyer’s or its designee’s balance account with DTC through its Deposit/Withdrawal at Custodian system or (B) if the Transfer Agent is not participating in FAST, issue and deliver (via reputable overnight courier) to such Buyer, a certificate representing such Securities that is free from all restrictive and other legends, registered in the name of such Buyer or its designee (the date by which such credit is so required to be made to the balance account of such Buyer’s or such Buyer’s designee with DTC or such certificate is required to be delivered to such Buyer pursuant to the foregoing is referred to herein as the “Required Delivery Date”, and the date such shares of Common Stock are actually delivered without restrictive legend to such Buyer or such Buyer’s designee with DTC, as applicable, the “Share Delivery Date”). The Company shall be responsible for any transfer agent fees or DTC fees with respect to any issuance of Securities or the removal of any legends with respect to any Securities in accordance herewith.
(e) Failure to Timely Deliver; Buy-In. If the Company fails, for any reason or for no reason, to issue and deliver (or cause to be delivered) to a Buyer (or its designee) by the Required Delivery Date, either (I) if the Transfer Agent is not participating in FAST, a certificate for the number of Conversion Shares to which such Buyer is entitled and register such Conversion Shares on the Company’s share register or, if the Transfer Agent is participating in FAST, to credit the balance account of such Buyer or such Buyer’s designee with DTC for such number of Conversion Shares submitted for legend removal by such Buyer pursuant to Section 5(d) above or (II) if the Registration Statement covering the resale of the Conversion Shares submitted for legend removal by such Buyer pursuant to Section 5(d)
Annex B-31
above (the “Unavailable Shares”) is not available for the resale of such Unavailable Shares and the Company fails to promptly, but in no event later than as required pursuant to the Registration Rights Agreement (x) so notify such Buyer and (y) deliver the Conversion Shares electronically without any restrictive legend by crediting such aggregate number of Conversion Shares submitted for legend removal by such Buyer pursuant to Section 5(d) above to such Buyer’s or its designee’s balance account with DTC through its Deposit/Withdrawal At Custodian system (the event described in the immediately foregoing clause (II) is hereinafter referred as a “Notice Failure” and together with the event described in clause (I) above, a “Delivery Failure”), then, in addition to all other remedies available to such Buyer, the Company shall pay in cash to such Buyer on each day after the Share Delivery Date and during such Delivery Failure an amount equal to 1% of the product of (A) the sum of the number of shares of Common Stock not issued to such Buyer on or prior to the Required Delivery Date and to which such Buyer is entitled, and (B) any trading price of the Common Stock selected by such Buyer in writing as in effect at any time during the period beginning on the date of the delivery by such Buyer to the Company of the applicable Conversion Shares and ending on the applicable Share Delivery Date. In addition to the foregoing, if on or prior to the Required Delivery Date either (I) if the Transfer Agent is not participating in FAST, the Company shall fail to issue and deliver a certificate to a Buyer and register such shares of Common Stock on the Company’s share register or, if the Transfer Agent is participating in FAST, credit the balance account of such Buyer or such Buyer’s designee with DTC for the number of shares of Common Stock to which such Buyer submitted for legend removal by such Buyer pursuant to Section 5(d) above (ii) below or (II) a Notice Failure occurs, and if on or after such Trading Day such Buyer acquires (in an open market transaction, stock loan or otherwise) shares of Common Stock corresponding to all or any portion of the number of shares of Common Stock submitted for legend removal by such Buyer pursuant to Section 5(d) above (a “Buy-In”), then the Company shall, within two (2) Trading Days after such Buyer’s request and in such Buyer’s discretion, either (i) pay cash to such Buyer in an amount equal to such Buyer’s total purchase price (including brokerage commissions, stock loan costs and other out-of-pocket expenses, if any) for the shares of Common Stock so acquired (including, without limitation, by any other Person in respect, or on behalf, of the holder) (the “Buy-In Price”), at which point the Company’s obligation to so deliver such certificate or credit such Buyer’s balance account shall terminate and such shares shall be cancelled, or (ii) promptly honor its obligation to so deliver to such Buyer a certificate or certificates or credit the balance account of such Buyer or such Buyer’s designee with DTC representing such number of shares of Common Stock that would have been so delivered if the Company timely complied with its obligations hereunder and pay cash to such Buyer in an amount equal to the excess (if any) of the Buy-In Price over the product of (A) such number of shares of Conversion Shares that the Company was required to deliver to such Buyer by the Required Delivery Date multiplied by (B) the lowest Closing Sale Price (as defined in the Notes) of the Common Stock on any Trading Day during the period commencing on the date of the delivery by such Buyer to the Company of the applicable Conversion Shares and ending on the date of such delivery and payment under this clause (ii). Nothing shall limit such Buyer’s right to pursue any other remedies available to it hereunder, at law or in equity, including, without limitation, a decree of specific performance and/or injunctive relief with respect to the Company’s failure to timely deliver certificates representing shares of Common Stock (or to electronically deliver such shares of Common Stock) as required pursuant to the terms hereof. Notwithstanding anything herein to the contrary, with respect to any given Notice Failure and/or Delivery Failure, this Section 5(e) shall not apply to the applicable Buyer the extent the Company has already paid such amounts in full to such Buyer with respect to such Notice Failure and/or Delivery Failure, as applicable, pursuant to the analogous sections of the Note held by such Buyer.
(f) FAST Compliance. While any Notes remain outstanding, the Company shall maintain a transfer agent that participates in FAST.
6. CONDITIONS TO THE COMPANY’S OBLIGATION TO SELL.
(a) The obligation of the Company hereunder to issue and sell the Initial Notes to each Buyer at the Initial Closing is subject to the satisfaction, at or before the Initial Closing Date, of each of the following conditions, provided that these conditions are for the Company’s sole benefit and may be waived by the Company at any time in its sole discretion by providing each Buyer with prior written notice thereof:
(i) Such Buyer shall have executed each of the other Transaction Documents to which it is a party and delivered the same to the Company.
Annex B-32
(ii) Such Buyer and each other Buyer shall have delivered to the Company the Initial Purchase Price (less, in the case of any Buyer, the amounts withheld pursuant to Section 4(g)) for the Initial Note being purchased by such Buyer at the Initial Closing by wire transfer of immediately available funds in accordance with the Initial Flow of Funds Letter.
(iii) The representations and warranties of such Buyer shall be true and correct in all material respects as of the date when made and as of the Initial Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date), and such Buyer shall have performed, satisfied and complied in all material respects with the covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by such Buyer at or prior to the Initial Closing Date.
(b) The obligation of the Company hereunder to issue and sell the Additional Notes to each Buyer at each Additional Closing is subject to the satisfaction, at or before the applicable Additional Closing Date, of each of the following conditions, provided that these conditions are for the Company’s sole benefit and may be waived by the Company at any time in its sole discretion by providing each Buyer with prior written notice thereof:
(i) Such Buyer shall have executed each of the other Transaction Documents to which it is a party and delivered the same to the Company.
(ii) Such Buyer and each other Buyer shall have delivered to the Company the Additional Purchase Price (less, in the case of any Buyer, the amounts withheld pursuant to Section 4(g)) for the Additional Note being purchased by such Buyer at the Additional Closing by wire transfer of immediately available funds in accordance with the Additional Flow of Funds Letter.
(iii) The representations and warranties of such Buyer shall be true and correct in all material respects as of the date when made and as of the Additional Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date), and such Buyer shall have performed, satisfied and complied in all material respects with the covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by such Buyer at or prior to the Additional Closing Date.
7. CONDITIONS TO EACH BUYER’S OBLIGATION TO PURCHASE.
(a) The obligation of each Buyer hereunder to purchase its Initial Note at the Initial Closing is subject to the satisfaction, at or before the Initial Closing Date, of each of the following conditions, provided that these conditions are for each Buyer’s sole benefit and may be waived by such Buyer at any time in its sole discretion by providing the Company with prior written notice thereof:
(i) The Company shall have duly executed and delivered to such Buyer each of the Transaction Documents to which it is a party and the Company shall have duly executed and delivered to such Buyer an Initial Note in such original principal amount as is set forth across from such Buyer’s name in column (3) of the Schedule of Buyers, as being purchased by such Buyer at the Initial Closing pursuant to this Agreement.
(ii) Such Buyer shall have received the opinion of Nelson Mullins Riley & Scarborough LLP, the Company’s counsel, dated as of the Initial Closing Date, in the form acceptable to such Buyer.
(iii) The Company shall have delivered to such Buyer a copy of the Irrevocable Transfer Agent Instructions, in the form acceptable to such Buyer, which instructions shall have been delivered to and acknowledged in writing by the Transfer Agent and shall remain in full force and effect as of such Initial Closing Date.
(iv) The Company shall have delivered to such Buyer a certificate evidencing the formation and good standing of the Company in such entity’s jurisdiction of formation issued by the Secretary of State (or comparable office) of such jurisdiction of formation as of a date within thirty (30) days of the Initial Closing Date.
Annex B-33
(v) The Company shall have delivered to such Buyer a certificate evidencing the Company’s qualification as a foreign corporation and good standing issued by the Secretary of State (or comparable office) of each jurisdiction in which the Company conducts business and is required to so qualify, as of a date within thirty (30) days of the Initial Closing Date.
(vi) The Company shall have delivered to such Buyer a certificate, in the form acceptable to such Buyer, executed by the Secretary of the Company dated as of the Initial Closing Date, as to (i) the resolutions consistent with Section 3(b) as adopted by the Company’s board of directors in a form reasonably acceptable to such Buyer, (ii) the Certificate of Incorporation of the Company and (iii) the Bylaws of the Company, each as in effect at the Initial Closing.
(vii) Each and every representation and warranty of the Company shall be true and correct as of the date when made and as of the Initial Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date) and the Company shall have performed, satisfied and complied in all respects with the covenants, agreements and conditions required to be performed, satisfied or complied with by the Company at or prior to the Initial Closing Date. Such Buyer shall have received a certificate, duly executed by the Chief Executive Officer of the Company, dated as of the Initial Closing Date, to the foregoing effect and as to such other matters as may be reasonably requested by such Buyer in the form acceptable to such Buyer.
(viii) The Company shall have delivered to such Buyer a letter from the Transfer Agent certifying the number of shares of Common Stock outstanding on the Initial Closing Date immediately prior to the Initial Closing.
(ix) The Common Stock (A) shall be designated for quotation or listed (as applicable) on the Principal Market and (B) shall not have been suspended, as of the Initial Closing Date, by the SEC or the Principal Market from trading on the Principal Market nor shall suspension by the SEC or the Principal Market have been threatened, as of the Initial Closing Date, either (I) in writing by the SEC or the Principal Market or (II) by falling below the minimum maintenance requirements of the Principal Market.
(x) The Company shall have obtained all governmental, regulatory or third-party consents and approvals, if any, necessary for the sale of the Securities, including without limitation, those required by the Principal Market, if any.
(xi) No statute, rule, regulation, executive order, decree, ruling or injunction shall have been enacted, entered, promulgated or endorsed by any court or Governmental Entity of competent jurisdiction that prohibits the consummation of any of the transactions contemplated by the Transaction Documents.
(xii) Since the date of execution of this Agreement, no event or series of events shall have occurred that reasonably would have or result in a Material Adverse Effect.
(xiii) The Company shall have obtained approval of the Principal Market to list or designate for quotation (as the case may be) the Initial Conversion Shares.
(xiv) The Company shall have duly executed and delivered to such Buyer a voting agreement, in the form of Exhibit C hereof (each, a “Voting Agreement”), by and between the Company and each of the shareholders listed on Schedule 7(a)(xiv) attached hereto (collectively, the “Shareholders”) and each of the Shareholders, severally, shall have duly executed and delivered to such Buyer a Voting Agreement and the Stockholder Consent.
(xv) Prior to the time of consummation of the Initial Closing, the Transactions shall be consummated.
(xvi) Such Buyer shall have received a letter on the letterhead of the Company (the “Initial Flow of Funds Letter”) duly executed by the Chief Executive Officer of the Company, setting forth the wire amounts of each Buyer and the wire transfer instructions of the Company.
(xvii) No bona fide dispute shall exist, by and between (or among) any of the Buyers, any holder of Notes and/or the Company, which dispute is reasonably related to this Agreement, any of the Securities and/or the transactions contemplated hereby or thereby, as applicable.
Annex B-34
(xviii) The letter agreement, by and among the Company, Lokahi Therapeutics, Inc., a Nevada corporation and MindWave Innovations Inc, a Delaware corporation (as in effect as of the Initial Closing Date, the “Side Letter”), shall be in form and substance satisfactory to such Buyer in its sole discretion and, other than the Side Letter, no other agreement shall amend, modify or waive the transactions contemplated thereby.
(xix) The Company and its Subsidiaries shall have delivered to such Buyer such other documents, instruments or certificates relating to the transactions contemplated by this Agreement as such Buyer or its counsel may reasonably request.
(b) The obligation of each Buyer hereunder to purchase the applicable Additional Note at any Additional Closing is subject to the satisfaction, at or before the applicable Additional Closing Date, of each of the following conditions, provided that these conditions are for each Buyer’s sole benefit and may be waived by such Buyer at any time in its sole discretion by providing the Company with prior written notice thereof:
(i) The Company shall have duly executed and delivered to such Buyer each of the Transaction Documents to which it is a party and the Company shall have duly executed and delivered to each applicable Transaction Documents to which it is a party and the Company shall have duly executed and delivered to such Buyer such Additional Note being purchased by such Buyer at such Additional Closing pursuant to this Agreement.
(ii) Such Buyer shall have received the opinion of Duane Morris LLP, the Company’s counsel, dated as of such Additional Closing Date, in the form acceptable to such Buyer.
(iii) The Company shall have delivered to such Buyer a copy of the Irrevocable Transfer Agent Instructions, in the form acceptable to such Buyer, which instructions shall have been delivered to and acknowledged in writing by the Company’s transfer agent and shall remain in full force and effect as of such Additional Closing Date.
(iv) The Company shall have delivered to such Buyer a certificate evidencing the formation and good standing (if a good standing concept exists in such jurisdiction) of the Company in such entity’s jurisdiction of formation issued by the Secretary of State (or comparable office) of such jurisdiction of formation as of a date within ten (10) days of such Additional Closing Date.
(v) The Company shall have delivered to such Buyer a certificate evidencing the Company’s qualification as a foreign corporation and good standing issued by the Secretary of State (or comparable office) of each jurisdiction in which the Company conducts business and is required to so qualify, as of a date within ten (10) days of such Additional Closing Date.
(vi) The Company shall have delivered to such Buyer a certified copy of the Certificate of Incorporation as certified by the Delaware Secretary of State within ten (10) days of the Additional Closing Date.
(vii) The Company shall have delivered to such Buyer a certificate, in the form acceptable to such Buyer, executed by the Secretary of the Company and dated as of such Additional Closing Date, as to (i) the resolutions consistent with Section 3(b) as adopted by the Company’s board of directors in a form reasonably acceptable to such Buyer, (ii) the Certificate of Incorporation of the Company and (iii) the Bylaws of the Company, each as in effect at such Additional Closing.
(viii) Each and every representation and warranty of the Company shall be true and correct as of the date when made and as of such Additional Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date) and the Company shall have performed, satisfied and complied in all respects with the covenants, agreements and conditions required to be performed, satisfied or complied with by the Company at or prior to such Additional Closing Date. Such Buyer shall have received a certificate, duly executed by the Chief Executive Officer of the Company, dated as of such Additional Closing Date, to the foregoing effect and as to such other matters as may be reasonably requested by such Buyer in the form acceptable to such Buyer.
Annex B-35
(ix) The Company shall have delivered to such Buyer a letter from the Company’s transfer agent certifying the number of shares of Common Stock outstanding on such Additional Closing Date immediately prior to such Additional Closing.
(x) The Common Stock (A) shall be designated for quotation or listed (as applicable) on the Principal Market and (B) shall not have been suspended, as of such Additional Closing Date, by the SEC or the Principal Market from trading on the Principal Market nor shall suspension by the SEC or the Principal Market have been threatened, as of such Additional Closing Date, either (I) in writing by the SEC or the Principal Market or (II) by falling below the minimum maintenance requirements of the Principal Market.
(xi) The Company shall have obtained all governmental, regulatory or third-party consents and approvals, if any, necessary for the sale of the Securities, including without limitation, those required by the Principal Market, if any.
(xii) No statute, rule, regulation, executive order, decree, ruling or injunction shall have been enacted, entered, promulgated or endorsed by any court or Governmental Entity of competent jurisdiction that prohibits the consummation of any of the transactions contemplated by the Transaction Documents.
(xiii) Since the date of execution of this Agreement, no event or series of events shall have occurred that reasonably would have or result in a Material Adverse Effect.
(xiv) The Company shall have obtained approval of the Principal Market to list or designate for quotation (as the case may be) the Conversion Shares issuable upon conversion of such Additional Notes to be sold in such Additional Closing (without regard to any limitations on conversion set forth therein).
(xv) Such Buyer shall have received a letter on the letterhead of the Company (the “Additional Flow of Funds Letter”) duly executed by the Chief Financial Officer of the Company, setting forth the wire amounts of each Buyer and the wire transfer instructions of the Company.
(xvi) The Stockholder Approval shall have been obtained.
(xvii) No bona fide dispute shall exist, by and between (or among) any of the Buyers, any holder of Notes and/or the Company, which dispute is reasonably related to this Agreement, any of the Securities and/or the transactions contemplated hereby or thereby, as applicable.
(xviii) The Company and its Subsidiaries shall have delivered to such Buyer such other documents, instruments or certificates relating to the transactions contemplated by this Agreement as such Buyer or its counsel may reasonably request.
8. TERMINATION.
In the event that the Initial Closing shall not have occurred with respect to a Buyer within five (5) days of the date hereof, then such Buyer shall have the right to terminate its obligations under this Agreement with respect to itself at any time on or after the close of business on such date without liability of such Buyer to any other party; provided, however, (i) the right to terminate this Agreement under this Section 8 shall not be available to such Buyer if the failure of the transactions contemplated by this Agreement to have been consummated by such date is the result of such Buyer’s breach of this Agreement and (ii) the abandonment of the sale and purchase of the Notes shall be applicable only to such Buyer providing such written notice, provided further that no such termination shall affect any obligation of the Company under this Agreement to reimburse such Buyer for the expenses described in Section 4(g) above. Nothing contained in this Section 8 shall be deemed to release any party from any liability for any breach by such party of the terms and provisions of this Agreement or the other Transaction Documents or to impair the right of any party to compel specific performance by any other party of its obligations under this Agreement or the other Transaction Documents.
9. MISCELLANEOUS.
(a) Governing Law; Jurisdiction; Jury Trial. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by the internal laws of the State of New York, without giving effect to any provision or rule (whether of the State of New York or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of New York. The Company hereby irrevocably submits
Annex B-36
to the exclusive jurisdiction of the state and federal courts sitting in The City of New York, Borough of Manhattan, for the adjudication of any dispute hereunder or in connection herewith or under any of the other Transaction Documents or with any transaction contemplated hereby or thereby, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. Nothing contained herein shall be deemed or operate to preclude any Buyer from bringing suit or taking other legal action against the Company in any other jurisdiction to collect on the Company’s obligations to such Buyer or to enforce a judgment or other court ruling in favor of such Buyer. EACH PARTY HEREBY IRREVOCABLY WAIVES ANY RIGHT IT MAY HAVE TO, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR UNDER ANY OTHER TRANSACTION DOCUMENT OR IN CONNECTION WITH OR ARISING OUT OF THIS AGREEMENT, ANY OTHER TRANSACTION DOCUMENT OR ANY TRANSACTION CONTEMPLATED HEREBY OR THEREBY.
(b) Counterparts. This Agreement may be executed in two or more identical counterparts, all of which shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party. In the event that any signature is delivered by facsimile transmission or by an e-mail which contains a portable document format (.pdf) file of an executed signature page, such signature page shall create a valid and binding obligation of the party executing (or on whose behalf such signature is executed) with the same force and effect as if such signature page were an original thereof.
(c) Headings; Gender. The headings of this Agreement are for convenience of reference and shall not form part of, or affect the interpretation of, this Agreement. Unless the context clearly indicates otherwise, each pronoun herein shall be deemed to include the masculine, feminine, neuter, singular and plural forms thereof. The terms “including,” “includes,” “include” and words of like import shall be construed broadly as if followed by the words “without limitation.” The terms “herein,” “hereunder,” “hereof” and words of like import refer to this entire Agreement instead of just the provision in which they are found.
(d) Severability; Maximum Payment Amounts. If any provision of this Agreement is prohibited by law or otherwise determined to be invalid or unenforceable by a court of competent jurisdiction, the provision that would otherwise be prohibited, invalid or unenforceable shall be deemed amended to apply to the broadest extent that it would be valid and enforceable, and the invalidity or unenforceability of such provision shall not affect the validity of the remaining provisions of this Agreement so long as this Agreement as so modified continues to express, without material change, the original intentions of the parties as to the subject matter hereof and the prohibited nature, invalidity or unenforceability of the provision(s) in question does not substantially impair the respective expectations or reciprocal obligations of the parties or the practical realization of the benefits that would otherwise be conferred upon the parties. The parties will endeavor in good faith negotiations to replace the prohibited, invalid or unenforceable provision(s) with a valid provision(s), the effect of which comes as close as possible to that of the prohibited, invalid or unenforceable provision(s). Notwithstanding anything to the contrary contained in this Agreement or any other Transaction Document (and without implication that the following is required or applicable), it is the intention of the parties that in no event shall amounts and value paid by the Company and/or any of its Subsidiaries (as the case may be), or payable to or received by any of the Buyers, under the Transaction Documents (including without limitation, any amounts that would be characterized as “interest” under applicable law) exceed amounts permitted under any applicable law. Accordingly, if any obligation to pay, payment made to any Buyer, or collection by any Buyer pursuant the Transaction Documents is finally judicially determined to be contrary to any such applicable law, such obligation to pay, payment or collection shall be deemed to have been made by mutual mistake of such Buyer, the Company and its Subsidiaries and such amount shall be deemed to have been adjusted with retroactive effect to the maximum amount or rate of interest, as the case may be, as would not be so prohibited by the applicable law. Such adjustment shall be effected, to the extent necessary, by reducing or refunding, at the option of such Buyer, the amount of interest or any other amounts which would constitute unlawful amounts required to be paid or actually paid to such Buyer under the Transaction Documents. For greater certainty, to the extent that any interest, charges, fees, expenses or other amounts required to be paid to or received by such Buyer under any of the Transaction Documents or related thereto are held to be within the meaning of “interest” or another applicable term to otherwise be violative of applicable law, such amounts shall be pro-rated over the period of time to which they relate.
Annex B-37
(e) Entire Agreement; Amendments. This Agreement, the other Transaction Documents and the schedules and exhibits attached hereto and thereto and the instruments referenced herein and therein supersede all other prior oral or written agreements between the Buyers, the Company, its Subsidiaries, their affiliates and Persons acting on their behalf, including, without limitation, any transactions by any Buyer with respect to Common Stock or the Securities, and the other matters contained herein and therein, and this Agreement, the other Transaction Documents, the schedules and exhibits attached hereto and thereto and the instruments referenced herein and therein contain the entire understanding of the parties solely with respect to the matters covered herein and therein; provided, however, nothing contained in this Agreement or any other Transaction Document shall (or shall be deemed to) (i) have any effect on any agreements any Buyer has entered into with, or any instruments any Buyer has received from, the Company or any of its Subsidiaries prior to the date hereof with respect to any prior investment made by such Buyer in the Company or (ii) waive, alter, modify or amend in any respect any obligations of the Company or any of its Subsidiaries, or any rights of or benefits to any Buyer or any other Person, in any agreement entered into prior to the date hereof between or among the Company and/or any of its Subsidiaries and any Buyer, or any instruments any Buyer received from the Company and/or any of its Subsidiaries prior to the date hereof, and all such agreements and instruments shall continue in full force and effect. Except as specifically set forth herein or therein, neither the Company nor any Buyer makes any representation, warranty, covenant or undertaking with respect to such matters. For clarification purposes, the Recitals are part of this Agreement. No provision of this Agreement may be amended other than by an instrument in writing signed by the Company and the Required Holders (as defined below), and any amendment to any provision of this Agreement made in conformity with the provisions of this Section 9(e) shall be binding on all Buyers and holders of Securities, as applicable; provided that no such amendment shall be effective to the extent that it (A) applies to less than all of the holders of the Securities then outstanding or (B) imposes any obligation or liability on any Buyer without such Buyer’s prior written consent (which may be granted or withheld in such Buyer’s sole discretion). No waiver shall be effective unless it is in writing and signed by an authorized representative of the waiving party, provided that the Required Holders may waive any provision of this Agreement, and any waiver of any provision of this Agreement made in conformity with the provisions of this Section 9(e) shall be binding on all Buyers and holders of Securities, as applicable, provided that no such waiver shall be effective to the extent that it (1) applies to less than all of the holders of the Securities then outstanding (unless a party gives a waiver as to itself only) or (2) imposes any obligation or liability on any Buyer without such Buyer’s prior written consent (which may be granted or withheld in such Buyer’s sole discretion). No consideration (other than reimbursement of legal fees) shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision of any of the Transaction Documents unless the same consideration also is offered to all of the parties to the Transaction Documents, all holders of the Notes. From the date hereof and while any Notes are outstanding, the Company shall not be permitted to receive any consideration from a Buyer or a holder of Notes that is not otherwise contemplated by the Transaction Documents in order to, directly or indirectly, induce the Company or any Subsidiary (i) to treat such Buyer or holder of Notes in a manner that is more favorable than to other similarly situated Buyers or holders of Notes, or (ii) to treat any Buyer(s) or holder(s) of Notes in a manner that is less favorable than the Buyer or holder of Notes that is paying such consideration; provided, however, that the determination of whether a Buyer has been treated more or less favorably than another Buyer shall disregard any securities of the Company purchased or sold by any Buyer. The Company has not, directly or indirectly, made any agreements with any Buyers relating to the terms or conditions of the transactions contemplated by the Transaction Documents except as set forth in the Transaction Documents. Without limiting the foregoing, the Company confirms that, except as set forth in this Agreement, no Buyer has made any commitment or promise or has any other obligation to provide any financing to the Company, any Subsidiary or otherwise. As a material inducement for each Buyer to enter into this Agreement, the Company expressly acknowledges and agrees that (x) no due diligence or other investigation or inquiry conducted by a Buyer, any of its advisors or any of its representatives shall affect such Buyer’s right to rely on, or shall modify or qualify in any manner or be an exception to any of, the Company’s representations and warranties contained in this Agreement or any other Transaction Document and (y) unless a provision of this Agreement or any other Transaction Document is expressly preceded by the phrase “except as disclosed in the SEC Documents,” nothing contained in any of the SEC Documents shall affect such Buyer’s right to rely on, or shall modify or qualify in any manner or be an exception to any of, the Company’s representations and warranties contained in this Agreement or any other Transaction Document. “Required Holders” means (I) prior to the Additional Closing Expiration Date, each Buyer entitled to purchase Notes at a Closing hereunder and (II) on or after the Additional Closing Expiration Date, holders of a majority of the Registrable Securities as of such time (excluding any Registrable Securities held by the Company or any of its Subsidiaries as of such time) issued or issuable hereunder or pursuant to the Notes (or the Buyers, with respect to any waiver or amendment of Section 4(o)).
Annex B-38
(f) Notices. Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing and will be deemed to have been delivered: (i) upon receipt, when delivered personally; (ii) upon receipt, when sent by electronic mail (provided that such sent email is kept on file (whether electronically or otherwise) by the sending party and the sending party does not receive an automatically generated message from the recipient’s email server that such e-mail could not be delivered to such recipient); or (iii) one (1) Business Day after deposit with an overnight courier service with next day delivery specified, in each case, properly addressed to the party to receive the same. The mailing addresses and e-mail addresses for such communications shall be:
On or before the Initial Closing, if to the Company:
Apimeds Pharmaceuticals US, Inc.
100 Matawan Road, Suite 325
New Jersey, NJ 07747
Attention: Erik Emerson
E-Mail: erik@apimedsus.com
On or before the Initial Closing, with a copy (for informational purposes only) to:
Nelson Mullins Riley & Scarborough LLP
301 Hillsborough Street, Suite 1400
Raleigh, NC 27603
Attn: David Mannheim; Mike Bradshaw
E-mail: david.mannheim@nelsonmullins.com; mike.bradshaw@nelsonmullins.com
Following the Initial Closing, if to the Company:
TechyTrade Innovations Pte Ltd
60 Paya Lebar Road.
#04-23
Singapore 409051
Attn: Vin Menon
Phone: 011 65- 8766-2816
E-mail: vin@mindwavedao.com
Following the Initial Closing, with a copy (for informational purposes only) to:
Duane Morris LLP
901 New York Avenue N.W., Suite 700 East
Washington, D.C. 20001-4795
Attention: Andrew M. Tucker
E-Mail: atucker@duanemorris.com
If to the Transfer Agent:
VStock Transfer, LLC
18 Lafayette Place
Woodmere, NY 11598
Telephone: (212) 828-8436
Attention: Young Kim
E-Mail: young@vstocktransfer.com; saction@vstocktransfer.com
Annex B-39
If to a Buyer, to its mailing address and e-mail address set forth on the Schedule of Buyers, with copies to such Buyer’s representatives as set forth on the Schedule of Buyers,
with a copy (for informational purposes only) to:
Kelley Drye & Warren LLP
3 World Trade Center
175 Greenwich Street
New York, NY 10007
Telephone: (212) 808-7540
Attention: Michael A. Adelstein, Esq.
E-mail: madelstein@kelleydrye.com
or to such other mailing address and/or e-mail address and/or to the attention of such other Person as the recipient party has specified by written notice given to each other party five (5) days prior to the effectiveness of such change, provided that Kelley Drye & Warren LLP shall only be provided copies of notices sent to the lead Buyer. Written confirmation of receipt (A) given by the recipient of such notice, consent, waiver or other communication, (B) mechanically or electronically generated by the sender’s e-mail containing the time, date and recipient’s e-mail or (C) provided by an overnight courier service shall be rebuttable evidence of personal service, receipt by e-mail or receipt from an overnight courier service in accordance with clause (i), (ii) or (iii) above, respectively.
(g) Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their respective successors and assigns, including any purchasers of any of the Notes. The Company shall not assign this Agreement or any rights or obligations hereunder without the prior written consent of the Required Holders, including, without limitation, by way of a Fundamental Transaction (as defined in the Notes) (unless the Company is in compliance with the applicable provisions governing Fundamental Transactions set forth in the Notes). A Buyer may assign some or all of its rights hereunder in connection with any transfer of any of its Securities without the consent of the Company, in which event such assignee shall be deemed to be a Buyer hereunder with respect to such assigned rights.
(h) No Third Party Beneficiaries. This Agreement is intended for the benefit of the parties hereto and their respective permitted successors and assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person, other than the Indemnitees (as defined below) referred to in Section 9(k).
(i) Survival. The representations, warranties, agreements and covenants shall survive each Closing. Each Buyer shall be responsible only for its own representations, warranties, agreements and covenants hereunder.
(j) Further Assurances. Each party shall do and perform, or cause to be done and performed, all such further acts and things, and shall execute and deliver all such other agreements, certificates, instruments and documents, as any other party may reasonably request in order to carry out the intent and accomplish the purposes of this Agreement and the consummation of the transactions contemplated hereby.
(k) Indemnification. In consideration of each Buyer’s execution and delivery of the Transaction Documents and acquiring the Securities thereunder and in addition to all of the Company’s other obligations under the Transaction Documents, the Company shall defend, protect, indemnify and hold harmless each Buyer and each holder of any Securities and all of their stockholders, partners, members, officers, directors, employees and direct or indirect investors and any of the foregoing Persons’ agents or other representatives (including, without limitation, those retained in connection with the transactions contemplated by this Agreement) (collectively, the “Indemnitees”) from and against any and all actions, causes of action, suits, claims, losses, costs, penalties, fees, liabilities and damages, and expenses in connection therewith (irrespective of whether any such Indemnitee is a party to the action for which indemnification hereunder is sought), and including reasonable attorneys’ fees and disbursements (the “Indemnified Liabilities”), incurred by any Indemnitee as a result of, or arising out of, or relating to (i) any misrepresentation or breach of any representation or warranty made by the Company or any Subsidiary in any of the Transaction Documents, (ii) any breach of any covenant, agreement or obligation of the Company or any Subsidiary contained in any of the Transaction Documents or (iii) any cause of action, suit, proceeding or claim brought or made against such Indemnitee by a third party (including for these purposes a derivative action brought on behalf of the Company or any Subsidiary) or which otherwise involves such Indemnitee that arises out of or results from (A) the execution, delivery, performance or enforcement of any of the Transaction Documents, (B) any transaction financed or to be financed in whole or in part,
Annex B-40
directly or indirectly, with the proceeds of the issuance of the Securities, (C) any disclosure properly made by such Buyer pursuant to Section 4(i), or (D) the status of such Buyer or holder of the Securities either as an investor in the Company pursuant to the transactions contemplated by the Transaction Documents or as a party to this Agreement (including, without limitation, as a party in interest or otherwise in any action or proceeding for injunctive or other equitable relief). To the extent that the foregoing undertaking by the Company may be unenforceable for any reason, the Company shall make the maximum contribution to the payment and satisfaction of each of the Indemnified Liabilities which is permissible under applicable law. Except as otherwise set forth herein, the mechanics and procedures with respect to the rights and obligations under this Section 9(k) shall be the same as those set forth in Section 6 of the Registration Rights Agreement.
(l) Construction. The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent, and no rules of strict construction will be applied against any party. No specific representation or warranty shall limit the generality or applicability of a more general representation or warranty. Each and every reference to share prices, shares of Common Stock and any other numbers in this Agreement that relate to the Common Stock shall be automatically adjusted for any stock splits, stock dividends, stock combinations, recapitalizations or other similar transactions that occur with respect to the Common Stock after the date of this Agreement. Notwithstanding anything in this Agreement to the contrary, for the avoidance of doubt, nothing contained herein shall constitute a representation or warranty against, or a prohibition of, any actions with respect to the borrowing of, arrangement to borrow, identification of the availability of, and/or securing of, securities of the Company in order for such Buyer (or its broker or other financial representative) to effect short sales or similar transactions in the future.
(m) Remedies. Each Buyer and in the event of assignment by Buyer of its rights and obligations hereunder, each holder of Securities, shall have all rights and remedies set forth in the Transaction Documents and all rights and remedies which such holders have been granted at any time under any other agreement or contract and all of the rights which such holders have under any law. Any Person having any rights under any provision of this Agreement shall be entitled to enforce such rights specifically (without posting a bond or other security), to recover damages by reason of any breach of any provision of this Agreement and to exercise all other rights granted by law. Furthermore, the Company recognizes that in the event that it or any Subsidiary fails to perform, observe, or discharge any or all of its or such Subsidiary’s (as the case may be) obligations under the Transaction Documents, any remedy at law would inadequate relief to the Buyers. The Company therefore agrees that the Buyers shall be entitled to specific performance and/or temporary, preliminary and permanent injunctive or other equitable relief from any court of competent jurisdiction in any such case without the necessity of proving actual damages and without posting a bond or other security. The remedies provided in this Agreement and the other Transaction Documents shall be cumulative and in addition to all other remedies available under this Agreement and the other Transaction Documents, at law or in equity (including a decree of specific performance and/or other injunctive relief).
(n) Withdrawal Right. Notwithstanding anything to the contrary contained in (and without limiting any similar provisions of) the Transaction Documents, whenever any Buyer exercises a right, election, demand or option under a Transaction Document and the Company or any Subsidiary does not timely perform its related obligations within the periods therein provided, then such Buyer may rescind or withdraw, in its sole discretion from time to time upon written notice to the Company or such Subsidiary (as the case may be), any relevant notice, demand or election in whole or in part without prejudice to its future actions and rights.
(o) Payment Set Aside; Currency. To the extent that the Company makes a payment or payments to any Buyer hereunder or pursuant to any of the other Transaction Documents or any of the Buyers enforce or exercise their rights hereunder or thereunder, and such payment or payments or the proceeds of such enforcement or exercise or any part thereof are subsequently invalidated, declared to be fraudulent or preferential, set aside, recovered from, disgorged by or are required to be refunded, repaid or otherwise restored to the Company, a trustee, receiver or any other Person under any law (including, without limitation, any bankruptcy law, foreign, state or federal law, common law or equitable cause of action), then to the extent of any such restoration the obligation or part thereof originally intended to be satisfied shall be revived and continued in full force and effect as if such payment had not been made or such enforcement or setoff had not occurred. Unless otherwise expressly indicated, all dollar amounts referred to in this Agreement and the other Transaction Documents are in United States Dollars (“U.S. Dollars”), and all amounts owing under this Agreement and all other Transaction Documents shall be paid in U.S. Dollars. All amounts denominated in other currencies (if any) shall be converted into the U.S. Dollar equivalent amount in accordance with
Annex B-41
the Exchange Rate on the date of calculation. “Exchange Rate” means, in relation to any amount of currency to be converted into U.S. Dollars pursuant to this Agreement, the U.S. Dollar exchange rate as published in the Wall Street Journal on the relevant date of calculation.
(p) Judgment Currency.
(i) If for the purpose of obtaining or enforcing judgment against the Company in connection with this Agreement or any other Transaction Document in any court in any jurisdiction it becomes necessary to convert into any other currency (such other currency being hereinafter in this Section 9(p) referred to as the “Judgment Currency”) an amount due in US Dollars under this Agreement, the conversion shall be made at the Exchange Rate prevailing on the Trading Day immediately preceding:
(1) the date actual payment of the amount due, in the case of any proceeding in the courts of New York or in the courts of any other jurisdiction that will give effect to such conversion being made on such date: or
(2) the date on which the foreign court determines, in the case of any proceeding in the courts of any other jurisdiction (the date as of which such conversion is made pursuant to this Section 9(p)(i)(2) being hereinafter referred to as the “Judgment Conversion Date”).
(ii) If in the case of any proceeding in the court of any jurisdiction referred to in Section 9(p)(i)(2) above, there is a change in the Exchange Rate prevailing between the Judgment Conversion Date and the date of actual payment of the amount due, the applicable party shall pay such adjusted amount as may be necessary to ensure that the amount paid in the Judgment Currency, when converted at the Exchange Rate prevailing on the date of payment, will produce the amount of US Dollars which could have been purchased with the amount of Judgment Currency stipulated in the judgment or judicial order at the Exchange Rate prevailing on the Judgment Conversion Date.
(iii) Any amount due from the Company under this provision shall be due as a separate debt and shall not be affected by judgment being obtained for any other amounts due under or in respect of this Agreement or any other Transaction Document.
(q) Independent Nature of Buyers’ Obligations and Rights. The obligations of each Buyer under the Transaction Documents are several and not joint with the obligations of any other Buyer, and no Buyer shall be responsible in any way for the performance of the obligations of any other Buyer under any Transaction Document. Nothing contained herein or in any other Transaction Document, and no action taken by any Buyer pursuant hereto or thereto, shall be deemed to constitute the Buyers as, and the Company acknowledges that the Buyers do not so constitute, a partnership, an association, a joint venture or any other kind of group or entity, or create a presumption that the Buyers are in any way acting in concert or as a group or entity, and the Company shall not assert any such claim with respect to such obligations or the transactions contemplated by the Transaction Documents or any matters, and the Company acknowledges that the Buyers are not acting in concert or as a group, and the Company shall not assert any such claim, with respect to such obligations or the transactions contemplated by the Transaction Documents. The decision of each Buyer to purchase Securities pursuant to the Transaction Documents has been made by such Buyer independently of any other Buyer. Each Buyer acknowledges that no other Buyer has acted as agent for such Buyer in connection with such Buyer making its investment hereunder and that no other Buyer will be acting as agent of such Buyer in connection with monitoring such Buyer’s investment in the Securities or enforcing its rights under the Transaction Documents. The Company and each Buyer confirms that each Buyer has independently participated with the Company and its Subsidiaries in the negotiation of the transaction contemplated hereby with the advice of its own counsel and advisors. Each Buyer shall be entitled to independently protect and enforce its rights, including, without limitation, the rights arising out of this Agreement or out of any other Transaction Documents, and it shall not be necessary for any other Buyer to be joined as an additional party in any proceeding for such purpose. The use of a single agreement to effectuate the purchase and sale of the Securities contemplated hereby was solely in the control of the Company, not the action or decision of any Buyer, and was done solely for the convenience of the Company and its Subsidiaries and not because it was required or requested to do so by any Buyer. It is expressly understood and agreed that each provision contained in this Agreement and in each other Transaction Document is between the Company, each Subsidiary and a Buyer, solely, and not between the Company, its Subsidiaries and the Buyers collectively and not between and among the Buyers.
[signature pages follow]
Annex B-42
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of the date first written above.
|
COMPANY: |
||||
|
APIMEDS PHARMACEUTICALS US, INC. |
||||
|
By: |
|
|||
|
Name: |
||||
|
Title: |
||||
Annex B-43
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Agreement to be duly executed as of the date first written above.
|
BUYER: |
||||
|
By: |
|
|||
|
Name: |
||||
|
Title: |
Annex B-44
SCHEDULE OF BUYERS
|
(1) |
(2) |
(3) |
(4) |
(5) |
(6) |
(7) |
(8) |
(8) |
|
Buyer |
Mailing |
Original Principal Amount of Initial Notes |
Aggregate Maximum Original Principal Amount of |
|
Aggregate Maximum Original Principal Amount of |
Initial Purchase Price |
Aggregate Maximum Additional Purchase Price |
Legal |
Annex B-45
AMENDMENT NO. 1 TO SECURITIES PURCHASE AGREEMENT
THIS AMENDMENT NO. 1 TO SECURITIES PURCHASE AGREEMENT (this “Amendment”) is dated as of December 8, 2025, by and among Apimeds Pharmaceuticals US, Inc., a Delaware corporation (the “Company”) and the undersigned (the “Investor”), and amends that certain Securities Purchase Agreement, dated as of December 1, 2025 (the “Securities Purchase Agreement”), by and among the Company and each of the investors listed on the Schedule of Buyers attached thereto. Capitalized terms used herein but not otherwise defined herein shall have the respective meanings set forth in the Securities Purchase Agreement.
WHEREAS, the Company and the Investor desire to amend certain provisions of the Securities Purchase Agreement pursuant to Section 9(e) thereof.
WHEREAS, pursuant to Section 9(e) of the Securities Purchase Agreement, the Company and the Required Holder may amend the terms of the Securities Purchase Agreement, which amendment shall be binding on all Buyers and holders of Securities.
NOW, THEREFORE, in consideration of the covenants and agreements contained therein, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Company and the Investor, intending to be legally bound, hereto agree as follows:
1. Amendments. As of the Effective Time (as defined below):
(a) Section 4(n) of the Securities Purchase Agreement is hereby amended to add the following:
“Until the later of (a) the thirty-six (36) month anniversary of the Initial Closing Date and (b) the Covenant Defeasance Date, the Company shall not enter into any other agreement that would prohibit or otherwise restrict any Subsequent Placement involving a Variable Rate Transaction with any Buyer (or any of its affiliates).”
(b) Section 4(o) of the Securities Purchase Agreement is hereby amended to add the following as clause (x):
“In the event that the Company fails to timely notify each Buyer of a Subsequent Placement in accordance with this Section 4(o), or otherwise fails to comply with any term or condition of this Section 4(o), then such Buyer shall have the right, at such Buyer’s option, at any time during the thirty (30) calendar day period commencing on the date of the Company’s initial public announcement of such Subsequent Placement, to deliver a written notice to the Company (which may be an e-mail), which written notice shall be deemed to be an Offer Notice for all purposes hereunder. Thereafter, such Buyer shall have the right to participate in such Subsequent Placement, and/or subscribe for the securities offered in such Subsequent Placement in a separate transaction, as applicable, under the terms of, and in compliance with, this Section 4(o) as if such Offer Notice had been delivered by the Company to such Buyer.
(c) The first sentence of Section 8 of the Securities Purchase Agreement is deleted in its entirety and replaced with the following sentence:
“In the event that the Initial Closing shall not have occurred with respect to a Buyer within ten (10) days of the date hereof, then such Buyer shall have the right to terminate its obligations under this Agreement with respect to itself at any time on or after the close of business on such date without liability of such Buyer to any other party; provided, however, (i) the right to terminate this Agreement under this Section 8 shall not be available to such Buyer if the failure of the transactions contemplated by this Agreement to have been consummated by such date is the result of such Buyer’s breach of this Agreement and (ii) the abandonment of the sale and purchase of the Notes shall be applicable only to such Buyer providing such written notice, provided further that no such termination shall affect any obligation of the Company under this Agreement to reimburse such Buyer for the expenses described in Section 4(g) above.”
(d) The definition of “Transaction Documents” in the Securities Purchase Agreement is hereby amended to include this Amendment.
Annex C-1
2. Acknowledgement; Ratification of Obligations. The Company and the Investor hereby confirm and agree that, except as set forth in Section 1 above, (i) the Securities Purchase Agreement and each other Transaction Documents are, and shall continue to be, in full force and effect, constitute legal and binding obligations of all parties thereto in accordance with its terms and are hereby ratified and confirmed in all respects, and (ii) the execution, delivery and effectiveness of this Amendment shall not operate as an amendment of any right, power or remedy of the Company or the Investor under any Transaction Document, nor constitute an amendment of any provision of any Transaction Document. This Amendment forms an integral and inseparable part of the Securities Purchase Agreement.
3. No Material Non-Public Information. Nothing in this Amendment constitutes material non-public information and the Company has previously disclosed all material, non-public information (if any) provided to the Investor by the Company or any of their respective officers, directors, employees or agents in connection with the transactions contemplated by hereby. The Company acknowledges and agrees that no confidentiality or similar obligations under any agreement, whether written or oral, between the Company, or any of their respective officers, directors, affiliates, employees or agents, on the one hand, and the Investor or any of its affiliates on the other hand, relating to the transactions contemplated hereby, exists as of the date hereof. Notwithstanding anything contained in this Amendment to the contrary and without implication that the contrary would otherwise be true, the Company expressly acknowledges and agrees that the Investor shall not have (unless expressly agreed to by the Investor after the date hereof in a written definitive and binding agreement executed by the Company and the Investor), any duty of confidentiality with respect to any material, non-public information regarding the Company.
4. Independent Nature of Investor’s Obligations and Rights. The obligations of the Investor under this Amendment or any other Transaction Document are several and not joint with the obligations of any other Buyer, and the Investor shall not be responsible in any way for the performance of the obligations of any other Buyer under any Transaction Document. Nothing contained herein or in any other Transaction Document, and no action taken by the Investor pursuant hereto, shall be deemed to constitute the Investor and other Buyers as a partnership, an association, a joint venture or any other kind of entity, or create a presumption that the Investor and other Buyers are in any way acting in concert or as a group with respect to such obligations or the transactions contemplated by this Amendment, or any other Transaction Document and the Company acknowledges that the Investor and the other Buyers are not acting in concert or as a group with respect to such obligations or the transactions contemplated by this Amendment, any other amendment and any other Transaction Document. The Company and the Investor confirm that the Investor has independently participated in the negotiation of the transactions contemplated hereby with the advice of its own counsel and advisors. The Investor shall be entitled to independently protect and enforce its rights, including, without limitation, the rights arising out of this Amendment, any other amendment or out of any other Transaction Documents, and it shall not be necessary for any other Buyers to be joined as an additional party in any proceeding for such purpose.
5. Effectiveness. Section 1 of this Amendment shall become effective upon the due execution and delivery by the Company and the Investor of this Amendment (the “Effective Time”).
6. References. As of the Effective Time, all references to the “Agreement” (including “hereof,” “herein,” “hereunder,” “hereby” and “this Agreement”) in the Securities Purchase Agreement and the other Transaction Documents shall refer to the Securities Purchase Agreement as amended by this Amendment. Notwithstanding the foregoing, references to the date of the Securities Purchase Agreement (as amended hereby) and references in the Securities Purchase Agreement to “the date hereof,” “the date of this Agreement” and terms of similar import shall in all instances continue to refer to December 1, 2025.
7. Miscellaneous. Section 9 of the Securities Purchase Agreement are hereby incorporated by reference herein, mutatis mutandis.
[THE REMAINDER OF THIS PAGE IS INTENTIONALLY LEFT BLANK.]
Annex C-2
IN WITNESS WHEREOF, the parties have caused their respective signature page to this Amendment to be duly executed as of the date first written above.
|
COMPANY: |
||||||
|
Apimeds Pharmaceuticals US, Inc. |
||||||
|
By: |
|
|||||
|
Name: |
Erik Emerson |
|||||
|
Title: |
President |
|||||
Annex C-3
IN WITNESS WHEREOF, the parties hereto have caused their respective signature page to this Amendment to be duly executed as of the date first written above.
|
INVESTOR: |
||||||
|
By: |
|
|||||
|
Name: |
||||||
|
Title: |
||||||
Annex C-4
AMENDMENT AND EXCHANGE AGREEMENT
This Amendment and Exchange Agreement (the “Agreement”) is entered into as of this 8th day of January, 2026, by and between Apimeds Pharmaceuticals US, Inc., a Delaware corporation with offices located at 100 Matawan Road, Suite 325, New Jersey, NJ 07747 (the “Company”) and the Holder signatory hereto (the “Holder”), with reference to the following facts:
A. The Holder is the holder of such aggregate original principal amount and interest of senior convertible notes as set forth on the signature page of Holder attached hereto (if any) (the “Holder Notes”). The Holder Notes were issued pursuant to that certain Securities Purchase Agreement, dated December 1, 2026, by and among the Holder, the Company and the other parties thereto (the “Securities Purchase Agreement”). Capitalized terms not defined herein shall have the meaning as set forth in the Securities Purchase Agreement.
B. The Company has authorized a new series of senior secured convertible notes of the Company, in the aggregate original principal amount of $120,900,000, substantially in the form attached hereto as Exhibit A-1 (the “Notes”), to be issued to the Holder pursuant to this Agreement.
C. The Company and the Holder desire to exchange (the “Exchange” or the “Transaction”) such aggregate outstanding amount of Holder Notes as set forth on the signature page of the Holder attached hereto (if any) (the “Existing Exchange Securities”) for such aggregate principal amount of Notes as set forth on the signature page of the Holder attached hereto (the “New Notes”). The New Notes, this Agreement and such other documents and certificates related thereto are collectively referred to herein as the “Exchange Documents”.
D. The Exchange is being made in reliance upon the exemption from registration provided by Section 3(a)(9) of the Securities Act of 1933, as amended (the “1933 Act”).
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants hereinafter contained, the parties hereto agree as follows:
1. Exchange. On the Closing Date, subject to the terms and conditions of this Agreement, the Holder shall, and the Company shall, pursuant to Section 3(a)(9) of the 1933 Act, exchange the Existing Exchange Securities for the New Notes.
2. Amendments. Effective as of the Closing Date, the Securities Purchase Agreement and each of the other Transaction Documents are hereby amended as follows (and any such agreements, covenants and related provisions therein shall be deemed incorporated by reference herein, mutatis mutandis, as amended as such):
2.1 The defined term “Notes” is hereby amended to include the New Notes (as defined herein).
2.2 Exhibit A-2 of the Securities Purchase Agreement is hereby amended and restated as Exhibit A hereof.
2.3 The defined term “Transaction Documents” is hereby amended to include this Agreement, the Intercreditor Agreement (as defined below) and the Controlled Account Agreement (as defined in the New Notes).
3. The Closing. Subject to the conditions set forth in Section 4.1 below, the Exchange shall take place via the electronic exchange of documents, securities and signatures, as of the Effective Time or at such other time and place as the Company and the Holder mutually agree (the “Closing” and the “Closing Date”).
4. Closing Conditions to The Closing.
4.1 Conditions to the Holder’s Obligations at the Closing. The obligation of the Holder to consummate the Exchange is subject to the fulfillment (or waiver, at the sole option of the Holder), to the Holder’s reasonable satisfaction, prior to or at the Closing, of each of the following conditions:
4.1.1 Representations and Warranties. Each and every representation and warranty of the Company set forth herein shall be true and correct in all material respects (except where qualified by materiality or Material Adverse Effect, which shall be true and correct in all respect) as of the date when made and as of the Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date) and the Company
Annex D-1
shall have performed, satisfied and complied in all respects with the covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by the Company at or prior to the Closing Date. The Holder shall have received a certificate, duly executed by the Chief Executive Officer of the Company, dated as of the Closing Date, to the foregoing effect and as to such other matters as may be reasonably requested by the Holder in the form acceptable to the Holder.
4.1.2 Issuance of Securities. At the Closing, the Company shall issue the New Notes on the books and records of the Company, with certificates with respect thereto delivered to the Holder no later than five (5) Trading Days after the Closing Date.
4.1.3 No Actions. No action, proceeding, investigation, regulation or legislation shall have been instituted, threatened or proposed before any court, governmental agency or authority or legislative body to enjoin, restrain, prohibit or obtain substantial damages in respect of, this Agreement or the consummation of the transactions contemplated by this Agreement.
4.1.4 Proceedings and Documents. All proceedings in connection with the transactions contemplated hereby and all documents and instruments incident to such transactions shall be satisfactory in substance and form to the Holder, and the Holder shall have received all such counterpart originals or certified or other copies of such documents as they may reasonably request. The Company shall have obtained all governmental, regulatory or third party consents and approvals, if any, necessary for the Exchange, including without limitation, those required by the Principal Market (defined hereafter), if any.
4.1.5 Listing. On each Trading Day during the twenty (20) Trading Days immediately preceding the Closing Date and the Closing Date, the Common Stock (I) shall be designated for quotation or listed on an Eligible Market (as defined in the New Notes) and (II) shall not have been suspended.
4.1.6 Additional Deliverables
(a) On or prior to the tenth (10th) Trading Day after the date hereof, the Company shall deliver to the Holder the Controlled Account Agreement (as defined in the New Notes), in form and substance satisfactory to the Holder, with respect to the Holder Control Account (as defined in the New Notes), which shall hold at least $8 million in U.S. dollars (or such lesser amount as permitted by the terms of the New Notes), duly executed by the Company and the applicable bank with respect thereto.
(b) On or prior to the tenth (10th) Trading Day after the date hereof, Lokahi Therapeutics, Inc. (“Lokahi”) and the Holder shall enter into an intercreditor agreement (the “Intercreditor Agreement”), in form and substance reasonably satisfactory to the Holder, but which shall include (i) an amendment to the Side Letter such that Lokahi shall receive up to a maximum of (x) $1 million upon the Applicable Date and (y) 50% of every dollar of principal converted under the Notes thereafter, and (ii) Lokahi shall be prohibited from accelerating any indebtedness (including, without limitation, any Indebtedness) or otherwise declaring any event of default with respect to any indebtedness (including, without limitation, any Indebtedness) until the 91st calendar day after such date no Notes remain outstanding.
(c) The Company shall have delivered to the Holder a certificate, in the form acceptable to the Holder, executed by the Secretary of the Company and dated as of the Closing Date, as to (i) the resolutions consistent with Section 7.1.2 below as adopted by the Company’s board of directors in a form reasonably acceptable to the Holder, (ii) the Certificate of Incorporation of the Company and (iii) the Bylaws (as defined below) of the Company, each as in effect at the Closing.
5. Conditions to the Company’s Obligations to the Closing. The obligation of the Company to consummate the Exchange is subject to the fulfillment (or waiver, at the sole option of the Company), to the Company’s reasonable satisfaction, prior to or at the Closing in question, of each of the following conditions:
5.1 Representations and Warranties. The representations and warranties of the Holder set forth herein shall be true and correct in all material respects as of the date when made and as of the Closing Date as though originally made at that time (except for representations and warranties that speak as of a specific date, which shall be true and correct as of such specific date), and the Holder shall have performed, satisfied and complied in all material respects with the covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by the Holder at or prior to the Closing Date.
Annex D-2
5.1.1 No Actions. No action, proceeding, investigation, regulation or legislation shall have been instituted, threatened or proposed before any court, governmental agency or authority or legislative body to enjoin, restrain, prohibit, or obtain substantial damages in respect of, this Agreement or the consummation of the transactions contemplated by this Agreement.
5.1.2 Proceedings and Documents. All proceedings in connection with the transactions contemplated hereby and all documents and instruments incident to such transactions shall be satisfactory in substance and form to the Company and the Company shall have received all such counterpart originals or certified or other copies of such documents as the Company may reasonably request.
6. Delivery of Securities; Ratifications; Payment; Effective Time.
6.1 Delivery of Securities
6.1.1 Delivery of Securities at Closing. On the Closing Date, the New Notes shall be issued on the books and records of the Company to the Holder. Within five (5) Trading Days after the Closing Date, the Company shall deliver the New Notes in certificate form to the Holder. Notwithstanding the foregoing, as of the Closing Date, the Holder shall be deemed for all corporate purposes to have become the holder of record of the New Notes and shall be entitled to exercise all of its rights with respect to the New Notes and, irrespective of the date the Company delivers such certificate evidencing the New Notes.
6.2 Legal Fee Payment. On or prior to the Closing Date, the Company shall pay, in U.S. dollars and immediately available funds, the Legal Fee Amount (as defined below) to Kelley Drye & Warren LLP.
6.3 Effective Time. This Agreement shall be deemed to be effective (the “Effective Time”) upon the time of full execution and delivery by the parties hereto of this Agreement and the other Exchange Documents.
6.4 Ratifications. Except as otherwise expressly provided herein, the Securities Purchase Agreement, and each other Transaction Document, is, and shall continue to be, in full force and effect and is hereby ratified and confirmed in all respects, except that on and after the date hereof: (i) all references in the Securities Purchase Agreement to “this Agreement”, “hereto”, “hereof”, “hereunder” or words of like import referring to the Securities Purchase Agreement shall mean the Securities Purchase Agreement as amended by this Agreement, and (ii) all references in the other Transaction Documents to the “Securities Purchase Agreement”, “thereto”, “thereof”, “thereunder” or words of like import referring to the Securities Purchase Agreement shall mean the Securities Purchase Agreement as amended by this Agreement.
7. Representations and Warranties.
7.1 Representations and Warranties of the Company. The Company represents and warrants to the Holder as of the date hereof and as of the Closing Date as follows:
7.1.1 Organization and Qualification. Each of the Company and each of its Subsidiaries are entities duly organized and validly existing and in good standing under the laws of the jurisdiction in which they are formed, and have the requisite power and authority to own their properties and to carry on their business as now being conducted and as presently proposed to be conducted. Each of the Company and each of its Subsidiaries is duly qualified as a foreign entity to do business and is in good standing in every jurisdiction in which its ownership of property or the nature of the business conducted by it makes such qualification necessary, except to the extent that the failure to be so qualified or be in good standing would not reasonably be expected to have a Material Adverse Effect (as defined below). As used in this Agreement, “Material Adverse Effect” means any material adverse effect on (i) the business, properties, assets, liabilities, operations (including results thereof), condition (financial or otherwise) or prospects of the Company or any Subsidiary, individually or taken as a whole, (ii) the transactions contemplated hereby or in any of the other Exchange Documents or (iii) the authority or ability of the Company or any of its Subsidiaries to perform any of their respective obligations under any of the Exchange Documents (as defined below). Other than the Persons (as defined below) listed in the SEC Documents, the Company has no Subsidiaries. “Subsidiaries” means any Person in which the Company, directly or indirectly, (I) owns any of the outstanding capital stock or holds any equity or similar interest of such Person or (II) controls or operates all or any part of the business,
Annex D-3
operations or administration of such Person, and each of the foregoing, is individually referred to herein as a “Subsidiary.” For purposes of this Agreement, (x) “Person” means an individual, a limited liability company, a partnership, a joint venture, a corporation, a trust, an unincorporated organization, any other entity and any Governmental Entity or any department or agency thereof and (y) “Governmental Entity” means any nation, state, county, city, town, village, district, or other political jurisdiction of any nature, federal, state, local, municipal, foreign, or other government, governmental or quasi-governmental authority of any nature (including any governmental agency, branch, department, official, or entity and any court or other tribunal), multi-national organization or body; or body exercising, or entitled to exercise, any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power of any nature or instrumentality of any of the foregoing, including any entity or enterprise owned or controlled by a government or a public international organization or any of the foregoing.
7.1.2 Authorization and Binding Obligation. The Company has the requisite power and authority to enter into and perform its obligations under this Agreement and each of the other agreements entered into by the parties hereto in connection with the transactions contemplated by the Exchange Documents and to consummate the Transaction (including, without limitation, the issuance of the New Notes in accordance with the terms hereof). The execution and delivery of the Exchange Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby, including, without limitation, the issuance of the New Notes in accordance with the terms hereof, has been duly authorized by the Company’s Board of Directors and no further filing, consent, or authorization is required by the Company, its Board of Directors or its stockholders. This Agreement and the other Exchange Documents have been duly executed and delivered by the Company, and constitute the legal, valid and binding obligations of the Company, enforceable against the Company in accordance with their respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies and except as rights to indemnification and to contribution may be limited by federal or state securities laws.
7.1.3 No Conflict. The execution, delivery and performance of the Exchange Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the New Notes in accordance with the terms hereof) will not (i) result in a violation of the Certificate of Incorporation or any other organizational documents of the Company or any of its Subsidiaries, any capital stock of the Company or any of its Subsidiaries or Bylaws of the Company or any of its Subsidiaries, (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Company or any of its Subsidiaries is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including foreign, federal and state securities laws and regulations and the rules and regulations of the Nasdaq Capital Market (the “Principal Market”) and including all applicable federal laws, rules and regulations) applicable to the Company or any of its Subsidiaries or by which any property or asset of the Company or any of its Subsidiaries is bound or affected except in the case of clauses (ii) and (iii) above, for such conflicts, defaults, rights or violations which would not, individually or in the aggregate, reasonably be expected to have a Material Adverse Effect on the ability of the Company to perform its obligations hereunder.
7.1.4 No Consents. Neither the Company nor any Subsidiary is required to obtain any consent from, authorization or order of, or make any filing or registration with any court, governmental agency or any regulatory or self-regulatory agency or any other Person in order for it to execute, deliver or perform any of its respective obligations under or contemplated by the Exchange Documents, in each case, in accordance with the terms hereof or thereof. All consents, authorizations, orders, filings and registrations which the Company or any Subsidiary is required to obtain pursuant to the preceding sentence have been or will be obtained or effected on or prior to the Closing Date, which shall be obtained, as otherwise provided in this Agreement, and neither the Company nor any of its Subsidiaries are aware of any facts or circumstances which might prevent the Company or any of its Subsidiaries from obtaining or effecting any of the registration, application or filings contemplated by the Exchange Documents. Except as disclosed in the SEC Documents, the Company is not in violation of the requirements of the Principal Market and has no knowledge of any facts or circumstances which would reasonably lead to delisting or suspension of the Common Stock in the foreseeable future.
Annex D-4
7.1.5 Securities Law Exemptions. Assuming the accuracy of the representations and warranties of the Holder contained herein, the offer and issuance by the Company of the New Notes in the Exchange is exempt from registration under the 1933 Act pursuant to the exemption provided by Rule 3(a)(9) thereof.
7.1.6 Issuance of New Notes. The issuance of the New Notes are duly authorized and, upon issuance in accordance with the terms of this Agreement, the New Notes shall be validly issued, fully paid and non-assessable and free from all preemptive or similar rights, mortgages, defects, claims, liens, pledges, charges, taxes, rights of first refusal, encumbrances, security interests and other encumbrances with respect to the issue thereof. Assuming the accuracy of the representations and warranties of the Holder contained herein, the offer and issuance by the Company of the New Notes is exempt from registration under the 1933 Act.
7.1.7 Transfer Taxes. On the date hereof, all share transfer or other taxes (other than income or similar taxes) which are required to be paid in connection with the issuance of the New Notes to be exchanged with the Holder hereunder will be, or will have been, fully paid or provided for by the Company, and all laws imposing such taxes will be or will have been complied with.
7.1.8 SEC Documents; Financial Statements. During the two (2) years prior to the date hereof, the Company has timely filed all reports, schedules, forms, proxy statements, statements and other documents required to be filed by it with the SEC pursuant to the reporting requirements of the 1934 Act (all of the foregoing filed prior to the date hereof, including without limitation, Current Reports on Form 8-K filed by the Company with the SEC whether required to be filed or not (but excluding Item 7.01 thereunder), and all exhibits and appendices included therein (other than Exhibits 99.1 to Form 8-K) and financial statements, notes and schedules thereto and documents incorporated by reference therein being hereinafter referred to as the “SEC Documents”). The Company has delivered or has made available to the Holder or its representatives true, correct and complete copies of each of the SEC Documents not available on the EDGAR system. As of their respective dates, the SEC Documents complied in all material respects with the requirements of the 1934 Act and the rules and regulations of the SEC promulgated thereunder applicable to the SEC Documents, and none of the SEC Documents, at the time they were filed with the SEC, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. As of their respective dates, the financial statements of the Company included in the SEC Documents complied in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto as in effect as of the time of filing. Such financial statements have been prepared in accordance with generally accepted accounting principles (“GAAP”), consistently applied, during the periods involved (except (i) as may be otherwise indicated in such financial statements or the notes thereto, or (ii) in the case of unaudited interim statements, to the extent they may exclude footnotes or may be condensed or summary statements) and fairly present in all material respects the financial position of the Company as of the dates thereof and the results of its operations and cash flows for the periods then ended (subject, in the case of unaudited statements, to normal year-end audit adjustments which will not be material, either individually or in the aggregate). No other information provided by or on behalf of the Company to the Holder which is not included in the SEC Documents (including, without limitation, information in the disclosure schedules to this Agreement) contains any untrue statement of a material fact or omits to state any material fact necessary in order to make the statements therein not misleading, in the light of the circumstance under which they are or were made. The Company is not currently contemplating to amend or restate any of the financial statements (including, without limitation, any notes or any letter of the independent accountants of the Company with respect thereto) included in the SEC Documents (the “Financial Statements”), nor is the Company currently aware of facts or circumstances which would require the Company to amend or restate any of the Financial Statements, in each case, in order for any of the Financials Statements to be in compliance with GAAP and the rules and regulations of the SEC. The Company has not been informed by its independent accountants that they recommend that the Company amend or restate any of the Financial Statements or that there is any need for the Company to amend or restate any of the Financial Statements.
7.1.9 Absence of Certain Changes. Except as set forth in the SEC Documents, since the date of the Company’s most recent audited financial statements contained in a Form 10-K, there has been no material adverse change and no material adverse development in the business, assets, liabilities, properties,
Annex D-5
operations (including results thereof), condition (financial or otherwise) or prospects of the Company or any of its Subsidiaries. Since the date of the Company’s most recent audited financial statements contained in a Form 10-K, neither the Company nor any of its Subsidiaries has (i) declared or paid any dividends, (ii) sold any assets, individually or in the aggregate, outside of the ordinary course of business or (iii) except as disclosed in the SEC Documents, made any capital expenditures, individually or in the aggregate, outside of the ordinary course of business. Neither the Company nor any of its Subsidiaries has taken any steps to seek protection pursuant to any law or statute relating to bankruptcy, insolvency, reorganization, receivership, liquidation or winding up, nor does the Company or any Subsidiary have any knowledge or reason to believe that any of their respective creditors intend to initiate involuntary bankruptcy proceedings or any actual knowledge of any fact which would reasonably lead a creditor to do so. The Company and its Subsidiaries, individually and on a consolidated basis, are not as of the date hereof, and after giving effect to the transactions contemplated hereby to occur on the date hereof, will not be Insolvent (as defined below). For the purpose of this Agreement (x) “Insolvent” means, (i) with respect to the Company and its Subsidiaries, on a consolidated basis, (A) the present fair saleable value of the Company’s and its Subsidiaries’ assets is less than the amount required to pay the Company’s and its Subsidiaries’ total Indebtedness (as defined below), (B) the Company and its Subsidiaries are unable to pay their debts and liabilities, subordinated, contingent or otherwise, as such debts and liabilities become absolute and matured or (C) the Company and its Subsidiaries intend to incur or believe that they will incur debts that would be beyond their ability to pay as such debts mature; and (ii) with respect to the Company and each Subsidiary, individually, (A) the present fair saleable value of the Company’s or such Subsidiary’s (as the case may be) assets is less than the amount required to pay its respective total Indebtedness, (B) the Company or such Subsidiary (as the case may be) is unable to pay its respective debts and liabilities, subordinated, contingent or otherwise, as such debts and liabilities become absolute and matured or (C) the Company or such Subsidiary (as the case may be) intends to incur or believes that it will incur debts that would be beyond its respective ability to pay as such debts mature; (y) “Indebtedness” of any Person means, without duplication (A) all indebtedness for borrowed money, (B) all obligations issued, undertaken or assumed as the deferred purchase price of property or services (including, without limitation, “capital leases” in accordance with GAAP) (other than trade payables entered into in the ordinary course of business consistent with past practice), (C) all reimbursement or payment obligations with respect to letters of credit, surety bonds and other similar instruments, (D) all obligations evidenced by notes, bonds, debentures or similar instruments, including obligations so evidenced incurred in connection with the acquisition of property, assets or businesses, (E) all indebtedness created or arising under any conditional sale or other title retention agreement, or incurred as financing, in either case with respect to any property or assets acquired with the proceeds of such indebtedness (even though the rights and remedies of the seller or bank under such agreement in the event of default are limited to repossession or sale of such property), (F) all monetary obligations under any leasing or similar arrangement which, in connection with GAAP, consistently applied for the periods covered thereby, is classified as a capital lease, (G) all indebtedness referred to in clauses (A) through (F) above secured by (or for which the holder of such indebtedness has an existing right, contingent or otherwise, to be secured by) any Lien upon or in any property or assets (including accounts and contract rights) owned by any Person, even though the Person which owns such assets or property has not assumed or become liable for the payment of such indebtedness, and (H) all Contingent Obligations in respect of indebtedness or obligations of others of the kinds referred to in clauses (A) through (G) above; and (z) “Contingent Obligation” means, as to any Person, any direct or indirect liability, contingent or otherwise, of that Person with respect to any Indebtedness, lease, dividend or other obligation of another Person if the primary purpose or intent of the Person incurring such liability, or the primary effect thereof, is to provide assurance to the obligee of such liability that such liability will be paid or discharged, or that any agreements relating thereto will be complied with, or that the holders of such liability will be protected (in whole or in part) against loss with respect thereto.
7.1.10 No Undisclosed Events, Liabilities, Developments or Circumstances. Except as set forth in the SEC Documents, no event, liability, development or circumstance has occurred or exists, or is reasonably expected to exist or occur with respect to the Company, any of its Subsidiaries or any of their respective businesses, properties, liabilities, prospects, operations (including results thereof) or condition (financial or otherwise), that (i) would be required to be disclosed by the Company under applicable securities laws on a registration statement on Form S-1 filed with the SEC relating to an issuance and sale by the Company of its Common Stock and which has not been publicly announced, (ii) would reasonably expected to have a Material Adverse Effect on the Holder’s investment hereunder or (iii) would reasonably be expected to have a Material Adverse Effect.
Annex D-6
7.1.11 Conduct of Business; Regulatory Permits. Neither the Company nor any of its Subsidiaries is in violation of any term of or in default under its Certificate of Incorporation, any certificate of designation, preferences or rights of any other outstanding series of preferred stock of the Company or any of its Subsidiaries or Bylaws or their organizational charter, certificate of formation, memorandum of association, articles of association, Certificate of Incorporation or bylaws, respectively. Except as set forth in the SEC Documents, neither the Company nor any of its Subsidiaries is in violation of any judgment, decree or order or any statute, ordinance, rule or regulation applicable to the Company or any of its Subsidiaries, and neither the Company nor any of its Subsidiaries will conduct its business in violation of any of the foregoing, except in all cases for possible violations which could not, individually or in the aggregate, have a Material Adverse Effect. Except as set forth in the SEC Documents or on Schedule 7.1.11, without limiting the generality of the foregoing, the Company is not in violation of any of the rules, regulations or requirements of the Principal Market and has no knowledge of any facts or circumstances that could reasonably lead to delisting or suspension of the Common Stock by the Principal Market in the foreseeable future. Except as set forth in the SEC Documents or described on Schedule 7.1.11, during the two years prior to the date hereof, (i) the Common Stock has been listed or designated for quotation on the Principal Market, (ii) trading in the Common Stock has not been suspended by the SEC or the Principal Market and (iii) the Company has received no communication, written or oral, from the SEC or the Principal Market regarding the suspension or delisting of the Common Stock from the Principal Market. The Company and each of its Subsidiaries possess all certificates, authorizations and permits issued by the appropriate regulatory authorities necessary to conduct their respective businesses, except where the failure to possess such certificates, authorizations or permits would not have, individually or in the aggregate, a Material Adverse Effect, and neither the Company nor any such Subsidiary has received any notice of proceedings relating to the revocation or modification of any such certificate, authorization or permit. There is no agreement, commitment, judgment, injunction, order or decree binding upon the Company or any of its Subsidiaries or to which the Company or any of its Subsidiaries is a party which has or would reasonably be expected to have the effect of prohibiting or materially impairing any business practice of the Company or any of its Subsidiaries, any acquisition of property by the Company or any of its Subsidiaries or the conduct of business by the Company or any of its Subsidiaries as currently conducted other than such effects, individually or in the aggregate, which have not had and would not reasonably be expected to have a Material Adverse Effect on the Company or any of its Subsidiaries.
7.1.12 Transactions With Affiliates. Except as set forth in the SEC Documents, no current or former employee, partner, director, officer or stockholder (direct or indirect) of the Company or its Subsidiaries, or any associate, or, to the knowledge of the Company, any affiliate of any thereof, or any relative with a relationship no more remote than first cousin of any of the foregoing, is presently, or has ever been, (i) a party to any transaction with the Company or its Subsidiaries (including any contract, agreement or other arrangement providing for the furnishing of services by, or rental of real or personal property from, or otherwise requiring payments to, any such director, officer or stockholder or such associate or affiliate or relative Subsidiaries (other than for ordinary course services as employees, consultants, officers or directors of the Company or any of its Subsidiaries)) or (ii) the direct or indirect owner of an interest in any corporation, firm, association or business organization which is a competitor, supplier or customer of the Company or its Subsidiaries (except for a passive investment (direct or indirect) in less than 5% of the common stock of a company whose securities are traded on or quoted through an Eligible Market), nor does any such Person receive income from any source other than the Company or its Subsidiaries which relates to the business of the Company or its Subsidiaries or should properly accrue to the Company or its Subsidiaries. No employee, officer, stockholder or director of the Company or any of its Subsidiaries or member of his or her immediate family is indebted to the Company or its Subsidiaries, as the case may be, nor is the Company or any of its Subsidiaries indebted (or committed to make loans or extend or guarantee credit) to any of them, other than (i) for payment of salary for services rendered, (ii) reimbursement for reasonable expenses incurred on behalf of the Company, and (iii) for other standard employee benefits made generally available to all employees or executives (including stock option agreements outstanding under any stock option plan approved by the Board of Directors of the Company).
Annex D-7
7.1.13 Equity Capitalization.
(a) Definitions:
(i) “Common Stock” means (x) the Company’s shares of common stock, $0.01 par value per share, and (y) any capital stock into which such common stock shall have been changed or any share capital resulting from a reclassification of such common stock.
(ii) “Preferred Stock” means (x) the Company’s blank check preferred stock, $0.01 par value per share, the terms of which may be designated by the board of directors of the Company in a certificate of designations and (y) any capital stock into which such preferred stock shall have been changed or any share capital resulting from a reclassification of such preferred stock (other than a conversion of such preferred stock into Common Stock in accordance with the terms of such certificate of designations).
(b) Authorized and Outstanding Capital Stock. As of the date hereof, the authorized capital stock of the Company consists of (A) 100 million shares of Common Stock, of which, 12,575,983 are issued and outstanding as of the date hereof and 151,934,832 are available for issuance pursuant to Convertible Securities (as defined below), in each case exercisable or exchangeable for, or convertible into, shares of Common Stock, and (B) 10 million shares of Preferred Stock, of which 7,477,017 shares are issued and outstanding. “Convertible Securities” means any capital stock or other security of the Company or any of its Subsidiaries that is at any time and under any circumstances directly or indirectly convertible into, exercisable or exchangeable for, or which otherwise entitles the holder thereof to acquire, any capital stock or other security of the Company (including, without limitation, Common Stock) or any of its Subsidiaries.
(c) Valid Issuance; Available Shares; Affiliates. All of such outstanding shares are duly authorized and have been, or upon issuance will be, validly issued and are fully paid and nonassessable. Schedule 7.1.13(c) sets forth the number of shares of Common Stock that are (A) reserved for issuance pursuant to Convertible Securities and (B) that are, as of the date hereof, owned by Persons who are “affiliates” (as defined in Rule 405 of the 1933 Act and calculated based on the assumption that only officers, directors and holders of at least 10% of the Company’s issued and outstanding Common Stock are “affiliates” without conceding that any such Persons are “affiliates” for purposes of federal securities laws) of the Company or any of its Subsidiaries.
(d) Existing Securities; Obligations. Except as disclosed in the SEC Documents: (A) none of the Company’s or any Subsidiary’s shares, interests or capital stock is subject to from all preemptive or similar rights, mortgages, defects, claims, liens, pledges, charges, taxes, rights of first refusal, encumbrances, security interests and other encumbrances (collectively “Liens”) suffered or permitted by the Company or any Subsidiary; (B) there are no outstanding options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible into, or exercisable or exchangeable for, any shares, interests or capital stock of the Company or any of its Subsidiaries, or contracts, commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to issue additional shares, interests or capital stock of the Company or any of its Subsidiaries or options, warrants, scrip, rights to subscribe to, calls or commitments of any character whatsoever relating to, or securities or rights convertible into, or exercisable or exchangeable for, any shares, interests or capital stock of the Company or any of its Subsidiaries; (C) there are no agreements or arrangements under which the Company or any of its Subsidiaries is obligated to register the sale of any of their securities under the 1933 Act; (D) there are no outstanding securities or instruments of the Company or any of its Subsidiaries which contain any redemption or similar provisions, and there are no contracts, commitments, understandings or arrangements by which the Company or any of its Subsidiaries is or may become bound to redeem a security of the Company or any of its Subsidiaries; (E) there are no securities or instruments containing anti-dilution or similar provisions that will be triggered by the issuance of the New Notes; and (F) neither the Company nor any Subsidiary has any stock appreciation rights or “phantom stock” plans or agreements or any similar plan or agreement.
(e) Organizational Documents. The Company has furnished to the Holder true, correct and complete copies of the Company’s Certificate of Incorporation, as amended and as in effect on the date hereof (the “Certificate of Incorporation”), and the Company’s bylaws, as amended and as in effect on the date hereof (the “Bylaws”), and the terms of all Convertible Securities and the material rights of the holders thereof in respect thereto.
Annex D-8
7.1.14 Indebtedness and Other Contracts. Neither the Company nor any of its Subsidiaries, (i) except as disclosed on Schedule 7.1.14 attached hereto, has any outstanding debt securities, notes, credit agreements, credit facilities or other agreements, documents or instruments evidencing Indebtedness of the Company or any of its Subsidiaries or by which the Company or any of its Subsidiaries is or may become bound, (ii) is a party to any contract, agreement or instrument, except as disclosed in the SEC Documents, the violation of which, or default under which, by the other party(ies) to such contract, agreement or instrument could reasonably be expected to result in a Material Adverse Effect, (iii) has any financing statements securing obligations in any amounts filed in connection with the Company or any of its Subsidiaries, except as disclosed in the SEC Documents; (iv) is in violation of any term of, or in default under, any contract, agreement or instrument relating to any Indebtedness, except where such violations and defaults would not result, individually or in the aggregate, in a Material Adverse Effect, or (v) is a party to any contract, agreement or instrument relating to any Indebtedness, the performance of which, in the judgment of the Company’s officers, has or is expected to have a Material Adverse Effect. Neither the Company nor any of its Subsidiaries have any liabilities or obligations required to be disclosed in the SEC Documents which are not so disclosed in the SEC Documents, other than those incurred in the ordinary course of the Company’s or its Subsidiaries’ respective businesses and which, individually or in the aggregate, do not or could not have a Material Adverse Effect.
7.1.15 Litigation. There is no action, suit, arbitration, proceeding, inquiry or investigation before or by the Principal Market, any court, public board, other Governmental Entity, self-regulatory organization or body pending or, to the knowledge of the Company, threatened against or affecting the Company or any of its Subsidiaries, the Common Stock or any of the Company’s or its Subsidiaries’ officers or directors that would reasonably be expected to have a Material Adverse Effect on the Company or its Subsidiaries, whether of a civil or criminal nature or otherwise, in their capacities as such, except as disclosed in the SEC Documents. No director, officer or employee of the Company or any of its Subsidiaries has willfully violated 18 U.S.C. §1519 or engaged in spoliation in reasonable anticipation of litigation. Without limitation of the foregoing, there has not been, and to the knowledge of the Company, there is not pending or contemplated, any investigation by the SEC involving the Company, any of its Subsidiaries or any current or former director or officer of the Company or any of its Subsidiaries. The SEC has not issued any stop order or other order suspending the effectiveness of any registration statement filed by the Company under the 1933 Act or the 1934 Act. Neither the Company nor any of its Subsidiaries is subject to any order, writ, judgment, injunction, decree, determination or award of any Governmental Entity.
7.1.16 Disclosure. The Company confirms that neither it nor any other Person acting on its behalf has provided the Holder or its agents or counsel with any information that constitutes or would reasonably be expected to constitute material, non-public information concerning the Company or any of its Subsidiaries, other than the existence of the transactions contemplated by this Agreement and the other Exchange Documents. The Company understands and confirms that the Holder will rely on the foregoing representations in effecting transactions in securities of the Company. All disclosure provided to the Holder regarding the Company and its Subsidiaries, their businesses and the transactions contemplated hereby, including the schedules to this Agreement, furnished by or on behalf of the Company or any of its Subsidiaries is true and correct and does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make the statements made therein, in the light of the circumstances under which they were made, not misleading. No event or circumstance has occurred or information exists with respect to the Company or any of its Subsidiaries or its or their business, properties, liabilities, prospects, operations (including results thereof) or conditions (financial or otherwise), which, under applicable law, rule or regulation, requires public disclosure at or before the date hereof or announcement by the Company but which has not been so publicly announced or disclosed.
7.2 Holder’s Representations and Warranties. As a material inducement to the Company to enter into this Agreement and consummate the Transaction, the Holder represents and warrants with and to the Company as of the date hereof and as of the Closing Date as follows:
7.2.1 Reliance on Exemptions. The Holder understands that the New Notes are being offered and exchanged in reliance on specific exemptions from the registration requirements of United States federal and state securities laws and that the Company is relying in part upon the truth and accuracy of, and the
Annex D-9
Holder’s compliance with, the representations, warranties, agreements, acknowledgments and understandings of the Holder set forth herein and in the Exchange Documents in order to determine the availability of such exemptions and the eligibility of the Holder to acquire the New Notes.
7.2.2 No Governmental Review. The Holder understands that no United States federal or state agency or any other government or governmental agency has passed on or made any recommendation or endorsement of the New Notes or the fairness or suitability of the investment in the New Notes nor have such authorities passed upon or endorsed the merits of the offering of the New Notes.
7.2.3 Validity; Enforcement. This Agreement and the Exchange Documents to which the Holder is a party have been duly and validly authorized, executed and delivered on behalf of the Holder and shall constitute the legal, valid and binding obligations of the Holder enforceable against the Holder in accordance with their respective terms, except as such enforceability may be limited by general principles of equity or to applicable bankruptcy, insolvency, reorganization, moratorium, liquidation and other similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies.
7.2.4 No Conflicts. The execution, delivery and performance by the Holder of this Agreement and the Exchange Documents to which the Holder is a party, and the consummation by the Holder of the transactions contemplated hereby and thereby will not (i) result in a violation of the organizational documents of the Holder or (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Holder is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws) applicable to the Holder, except in the case of clauses (ii) and (iii) above, for such conflicts, defaults, rights or violations which would not, individually or in the aggregate, reasonably be expected to have a material adverse effect on the ability of the Holder to perform its obligations hereunder.
7.2.5 Investment Risk; Sophistication. The Holder is acquiring the New Notes hereunder in the ordinary course of its business. The Holder has such knowledge, sophistication, and experience in business and financial matters so as to be capable of evaluation of the merits and risks of the prospective investment in the New Notes, and has so evaluated the merits and risk of such investment. The Holder is an “accredited Holder” as defined in Regulation D under the 1933 Act.
7.2.6 Ownership of Existing Exchange Securities. The Holder owns the Existing Exchange Securities free and clear of any Liens (other than the obligations pursuant to this Agreement and applicable securities laws).
8. Disclosure of Transaction. The Company shall, on or before 9:30 a.m., New York City Time, on or prior to the first business day after the date of this Agreement, file a Current Report on Form 8-K describing the terms of the transactions contemplated hereby in the form required by the 1934 Act and attaching the forms of the New Notes and this Agreement as an exhibit to such filing (including all attachments, the “8-K Filing”). From and after the filing of the 8-K Filing, the Company shall have disclosed all material, non-public information (if any) provided up to such time to the Holder by the Company or any of its Subsidiaries or any of their respective officers, directors, employees or agents. In addition, effective upon the filing of the 8-K Filing, the Company acknowledges and agrees that any and all confidentiality or similar obligations under any agreement with respect to the transactions contemplated by the Exchange Documents or as otherwise disclosed in the 8-K Filing, whether written or oral, between the Company, any of its Subsidiaries or any of their respective officers, directors, affiliates, employees or agents, on the one hand, and any of the Holder or any of their affiliates, on the other hand, shall terminate. Neither the Company, its Subsidiaries nor the Holder shall issue any press releases or any other public statements with respect to the transactions contemplated hereby, without the written prior approval of the other party; provided, however, the Company shall be entitled, without the prior approval of the Holder, to make a press release or other public disclosure with respect to such transactions (i) in substantial conformity with the 8-K Filing and contemporaneously therewith or (ii) as is required by applicable law and regulations (provided that in the case of clause (i) the Holder shall be consulted by the Company in connection with any such press release or other public disclosure prior to its release). Without the prior written consent of the Holder (which may be granted or withheld in the Holder’s sole discretion), except as required by applicable law, the Company shall not (and shall cause each of its Subsidiaries and affiliates to not) disclose the name of the Holder in any filing, announcement, release or otherwise.
Annex D-10
9. No Integration. None of the Company, its Subsidiaries, any of their affiliates, or any Person acting on their behalf shall, directly or indirectly, make any offers or sales of any security (as defined in the 1933 Act) or solicit any offers to buy any security or take any other actions, under circumstances that would require registration of any of the New Notes under the 1933 Act or cause this offering to be integrated with such offering or any prior offerings by the Company for purposes of Regulation D under the 1933 Act.
10. Fees. The Company shall reimburse Kelley Drye & Warren, LLP (counsel to the lead Holder) in (i) an aggregate non-accountable amount of $15,000 (the “Legal Fee Amount”) for costs and expenses incurred by it in connection with drafting and negotiation of the Exchange Documents and (ii) the reasonably and documented fees and expenses of Kelley Drye & Warren, LLP with respect to the drafting and negotiation of the Intercreditor Agreement and Controlled Account Agreement. Each party to this Agreement shall bear its own expenses in connection with the structuring, documentation, negotiation and closing of the transactions contemplated hereby, except as provided in the previous sentence and except that the Company shall be responsible for the payment of any placement agent’s fees, financial advisory fees, transfer agent fees, Depository Trust Company (“DTC”) fees relating to or arising out of the transactions contemplated hereby.
11. Holding Period. For the purposes of Rule 144, the Company acknowledges that the holding period of the New Notes may be tacked onto the holding period of the Existing Exchange Securities the Company agrees not to take a position contrary to this Section 11. The Company acknowledges and agrees that (assuming the Holder is not an affiliate of the Company) (i) the New Notes will be eligible to be resold pursuant to Rule 144 on June 1, 2026, (ii) the Company is not aware of any event reasonably likely to occur that would reasonably be expected to result in the New Notes becoming ineligible to be resold by the Holder pursuant to Rule 144 on June 1, 2026 and (iii) in connection with any resale of the New Notes pursuant to Rule 144, the Holder shall solely be required to provide reasonable assurances that such applicable New Notes are eligible for resale, assignment or transfer under Rule 144, which shall not include an opinion of Holder’s counsel. The Company shall be responsible for any transfer agent fees or DTC fees or legal fees of the Company’s counsel with respect to the removal of legends, if any.
12. Blue Sky. The Company shall make all filings and reports relating to the Exchange required under applicable securities or “Blue Sky” laws of the states of the United States following the date hereof, if any.
13. Miscellaneous.
13.1 Successors and Assigns. Except as otherwise provided herein, the terms and conditions of this Agreement shall inure to the benefit of and be binding upon the parties hereto and the respective successors and assigns of the parties. Nothing in this Agreement, express or implied, is intended to confer upon any party, other than the parties hereto or their respective successors and assigns, any rights, remedies, obligations or liabilities under or by reason of this Agreement, except as expressly provided in this Agreement.
13.2 Governing Law; Jurisdiction; Jury Trial. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by the internal laws of the State of Delaware, without giving effect to any choice of law or conflict of law provision or rule that would cause the application of the laws of any jurisdictions other than the State of Delaware. Each party hereby irrevocably submits to the exclusive jurisdiction of the state or federal courts sitting in Wilmington, Delaware, for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law. EACH PARTY HEREBY IRREVOCABLY WAIVES ANY RIGHT IT MAY HAVE, AND AGREES NOT TO REQUEST, A JURY TRIAL FOR THE ADJUDICATION OF ANY DISPUTE HEREUNDER OR IN CONNECTION WITH OR ARISING OUT OF THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY.
13.3 Titles and Subtitles. The titles and subtitles used in this Agreement are used for convenience only and are not to be considered in construing or interpreting this Agreement.
Annex D-11
13.4 Notices. Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing and will be deemed to have been delivered: (i) upon receipt, when delivered personally; (ii) upon delivery, when sent by facsimile (provided confirmation of transmission is mechanically or electronically generated and kept on file by the sending party) or by electronic mail; or (iii) one Trading Day after deposit with an overnight courier service, in each case properly addressed to the party to receive the same. The addresses, facsimile numbers and e-mail addresses for such communications shall be as set forth on the signature pages attached hereto or to such other address, facsimile number and/or e-mail address and/or to the attention of such other Person as the recipient party has specified by written notice given to each other party five (5) days prior to the effectiveness of such change. Written confirmation of receipt (A) given by the recipient of such notice, consent, waiver or other communication, (B) mechanically or electronically generated by the sender’s facsimile machine or e-mail containing the time, date, recipient facsimile number and an image of the first page of such transmission or (C) provided by an overnight courier service shall be rebuttable evidence of personal service, receipt by facsimile or receipt from an overnight courier service in accordance with clause (i), (ii) or (iii) above, respectively.
13.5 Finder’s Fees. Each party represents that it neither is nor will be obligated for any finders’ fee or commission in connection with this Transaction, including, without limitation any commission or other remuneration, paid or given directly or indirectly for soliciting the Exchange. The Company shall indemnify and hold harmless the Holder from any liability for any commission or compensation described in this Section 13.5 (and the costs and expenses of defending against such liability or asserted liability) for which the Company or any of its officers, employees or representatives is responsible.
13.6 Amendments and Waivers. Any term of this Agreement may be amended and the observance of any term of this Agreement may be waived (either generally or in a particular instance and either retroactively or prospectively), only with the written consent of the Company and the Holder.
13.7 Severability. If one or more provisions of this Agreement are held to be unenforceable under applicable law, such provision shall be excluded from this Agreement and the balance of the Agreement shall be interpreted as if such provision were so excluded and shall be enforceable in accordance with its terms so long as this Agreement as so modified continues to express, without material change, the original intentions of the parties as to the subject matter hereof and the prohibited nature, invalidity or unenforceability of the provision(s) in question does not substantially impair the respective expectations or reciprocal obligations of the parties or the practical realization of the benefits that would otherwise be conferred upon the parties. The parties will endeavor in good faith negotiations to replace the prohibited, invalid or unenforceable provision(s) with a valid provision(s), the effect of which comes as close as possible to that of the prohibited, invalid or unenforceable provision(s).
13.8 Entire Agreement. This Agreement together with the other Exchange Documents, represents the entire agreement and understandings between the parties concerning the Exchange and the other matters described herein and therein and supersedes and replaces any and all prior agreements and understandings solely with respect to the subject matter hereof and thereof. Except as expressly set forth herein, nothing herein shall amend, modify or waive any term or condition of the other Exchange Documents.
13.9 Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
13.10 Interpretation. Unless the context of this Agreement clearly requires otherwise, (a) references to the plural include the singular, the singular the plural, the part the whole, (b) references to any gender include all genders, (c) “including” has the inclusive meaning frequently identified with the phrase “but not limited to” and (d) references to “hereunder” or “herein” relate to this Agreement.
13.11 No Third Party Beneficiaries. This Agreement is intended for the benefit of the parties hereto and their respective permitted successors and assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person.
13.12 Survival. The representations, warranties and covenants of the Company and the Holder contained herein shall survive the consummation of the Exchange and the issuance and delivery of the New Notes.
Annex D-12
13.13 Further Assurances. Each party shall do and perform, or cause to be done and performed, all such further acts and things, and shall execute and deliver all such other agreements, certificates, instruments and documents, as any other party may reasonably request in order to carry out the intent and accomplish the purposes of this Agreement and the consummation of the transactions contemplated hereby.
13.14 No Strict Construction. The language used in this Agreement will be deemed to be the language chosen by the parties to express their mutual intent, and no rules of strict construction will be applied against any party.
13.15 No Transfer of Assets. The Company shall not transfer any cash or other assets held in PNC Bank, account number #[ ], which as of the time of execution of this Agreement holds at least $8 million in U.S. dollars, to any Person without the prior written consent of the Holder. The parties hereto acknowledge and agree that Section 12(p) of the New Notes shall govern the release of such cash and other assets and any written consents in connection therewith.
[The remainder of the page is intentionally left blank]
Annex D-13
IN WITNESS WHEREOF, Holders and the Company have executed this Agreement as of the date set forth on the first page of this Agreement.
|
COMPANY: |
||||
|
APIMEDS PHARMACEUTICALS US, INC. |
||||
|
By: |
|
|||
|
Name: |
||||
|
Title: |
||||
|
Address: |
||||
|
100 Matawan Rd, Suite 325 |
||||
Annex D-14
IN WITNESS WHEREOF, Holders and the Company have executed this Agreement as of the date set forth on the first page of this Agreement.
|
Aggregate Principal and Interest of |
HOLDER: |
|||||||||
|
$10,900,000 of principal and |
By: |
|
||||||||
|
Name: |
||||||||||
|
Title: |
||||||||||
|
Aggregate Principal of New Notes to |
||||||||||
|
Address: |
|
|||||||||
|
$10,900,000 of principal |
|
|||||||||
|
|
|
|||||||||
|
|
||||||||||
Annex D-15
APIMEDS PHARMACEUTICALS US, INC.
CERTIFICATE OF DESIGNATION OF PREFERENCES,
RIGHTS AND LIMITATIONS
OF
SERIES A CONVERTIBLE PREFERRED STOCK
Pursuant to Section 151 of the
General Corporation Law of the State of Delaware
THE UNDERSIGNED DOES HEREBY CERTIFY, on behalf of Apimeds Pharmaceuticals US, Inc., a Delaware corporation (the “Corporation”), that the following resolution was duly adopted by the Board of Directors of the Corporation (the “Board”), in accordance with the provisions of Section 151 of the General Corporation Law of the State of Delaware (the “DGCL”), pursuant to a unanimous written consent of the Board executed on November 29, 2025, in lieu of a meeting, which resolution provides for the creation of a series of the Corporation’s Preferred Stock, par value $0.01 per share, which is designated as “Series A Convertible Preferred Stock,” with the preferences, rights and limitations set forth therein relating to dividends, conversion, redemption, dissolution and distribution of assets of the Corporation.
WHEREAS, the Amended and Restated Certificate of Incorporation of the Corporation (as amended, the “Certificate of Incorporation”) authorizes the issuance of up to 10,000,000 shares of preferred stock, par value $0.01 per share, of the Corporation (“Preferred Stock”) in one or more series, and expressly authorizes the Board, subject to limitations prescribed by law, to provide, out of the unissued shares of Preferred Stock, for series of Preferred Stock, and, with respect to each such series, to establish and fix the number of shares to be included in any series of Preferred Stock and the designation, rights, preferences, powers, restrictions, and limitations of the shares of such series; and
WHEREAS it is the desire of the Board to establish and fix the number of shares to be included in a new series of Preferred Stock and the designation, rights, preferences, and limitations of the shares of such new series.
NOW, THEREFORE, BE IT RESOLVED, that pursuant to authority conferred upon the Board by the Certificate of Incorporation, (i) a series of Preferred Stock of the Corporation be, and hereby is authorized by the Board, (ii) the Board hereby authorizes the issuance of 7,263,865 shares of “Series A Convertible Preferred Stock”, and (iii) the Board hereby fixes the designations, powers, preferences and relative, participating, optional or other special rights, and the qualifications, limitations or restrictions thereof, of such shares of Preferred Stock, as follows:
TERMS OF SERIES A CONVERTIBLE PREFERRED STOCK
1. Defined Terms. For purposes hereof, the following terms shall have the following meanings:
“Board” has the meaning set forth in the Recitals.
“Business Day” means any day except any Saturday, any Sunday, any day which is a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Certificate of Incorporation” has the meaning set forth in the Recitals.
“Common Stock” means the common stock, par value $0.01 per share, of the Corporation.
“Conversion” has the meaning set forth in Section 7.2.
“Conversion Ratio” has the meaning set forth in Section 7.3.
“Conversion Share(s)” means the shares of Common Stock issuable upon conversion of the Series A Convertible Preferred Stock, as set forth in Section 7.2.
“Converted Stock” means the shares of Series A Convertible Preferred Stock that are converted into Conversion Shares upon the Automatic Conversion, as set forth in Section 7.4.
“Corporation” has the meaning set forth in the Preamble.
Annex E-1
“Effective Date” means the third Business Day following the satisfaction of the Required Approvals, as set forth in Section 7.2.
“Holder” means a holder of Series A Convertible Preferred Stock.
“Person” means an individual, corporation, partnership, joint venture, limited liability company, governmental authority, unincorporated organization, trust, association, or other entity.
“Required Approval” has the meaning set forth in Section 7.2.
“Series A Convertible Preferred Stock” has the meaning set forth in Section 2.
“Stockholder Approval” has the meaning set forth in Section 7.2.
“Subsidiary” means, with respect to any Person, any other Person of which a majority of the outstanding shares or other equity interests having the power to vote for directors or comparable managers are owned, directly or indirectly, by the first Person.
“Trading Market” means the NYSE American LLC.
“Trading Market Approval” has the meaning set forth in Section 7.2.
2. Designation. There shall be a series of Preferred Stock that shall be designated as “Series A Convertible Preferred Stock” (the “Series A Convertible Preferred Stock”) and the number of shares constituting such series shall be 7,263,865. The rights, preferences, powers, restrictions, and limitations of the Series A Convertible Preferred Stock shall be as set forth herein.
3. Voting. The Series A Convertible Preferred Stock shall have no voting rights.
4. Dividends. The Series A Convertible Preferred Stock shall be entitled to receive the same dividend or distribution as if the shares of Series A Convertible Preferred Stock had been converted into Common Stock immediately prior to the record date for such dividend or distribution.
5. Liquidation. The Series A Convertible Preferred Stock shall be entitled to share pro rata with the holders of Common Stock in any distribution of the remaining assets of the Corporation.
6. Redemption. The Series A Convertible Preferred Stock shall have no redemption rights.
7. Conversion.
7.1 Conversions at Option of Holder. The Series A Convertible Preferred Stock is not convertible at the election of the Holder.
7.2 Automatic Conversion. Effective as of 5:00 p.m. Eastern time on the date that is the third Business Day following the later of (i) the date on which the Corporation’s stockholders approve the conversion of the Series A Convertible Preferred Stock into shares of Common Stock (each share, a “Conversion Share”) in accordance with the listing rules of the Trading Market (the “Stockholder Approval”), and (ii) the date on which the Trading Market has approved any required new listing application, including any resulting from a change in control or Reverse Merger (as defined in Section 341 of the NYSE American Company Guide), such that (A) the Corporation satisfies all applicable continuing listing requirements of the Trading Market (or has been granted a grace period therefrom), (B) the Corporation has not received any notice of non-compliance from the Trading Market, and (C) the Conversion Shares have been approved for listing on the Trading Market (collectively, the “Trading Market Approval” and together with the Stockholder Approval, the “Required Approvals”), such date being referred to herein as the “Effective Date,” each share of Series A Convertible Preferred Stock then outstanding shall automatically, and without any action required by the Holder thereof, convert into a number of shares of Common Stock equal to the Conversion Ratio (as defined below) (the “Conversion”).
7.3 Conversion Ratio. The “Conversion Ratio” for each share of Series A Convertible Preferred Stock shall be twenty (20) shares of Common Stock issuable upon the Conversion of each share of Series A Convertible Preferred Stock, subject to adjustment as provided herein.
Annex E-2
7.4 Procedures for Conversion; Effect of Conversion. On the Effective Date, all outstanding shares of Series A Convertible Preferred Stock shall be converted into Common Stock, without any further action by the relevant Holder of such shares or the Corporation. The shares of Series A Convertible Preferred Stock that are converted in the Conversion are referred to as the “Converted Stock.” The Converted Stock shall be automatically cancelled and shall no longer be deemed outstanding as of the Effective Date, and all rights with respect to such shares shall immediately cease and terminate as of such time, other than the right of the Holder to receive Conversion Shares in exchange therefor. The Conversion Shares shall be issued in book entry form and shall be delivered to the Holders within two Business Days of the Effective Date. All shares of Common Stock issued hereunder by the Corporation shall be duly and validly issued, fully paid, and nonassessable, free and clear of all taxes, liens, charges, and encumbrances with respect to the issuance thereof.
7.5 Reservation of Stock. The Corporation shall take all such actions as may be necessary to assure that the Conversion Shares may be issued without violation of any applicable law or governmental regulation or any requirements of any domestic securities exchange upon which shares of Common Stock may be listed (except for official notice of issuance which shall be immediately delivered by the Corporation upon each such issuance). The Corporation shall not close its books against the transfer of any of its capital stock in any manner which would prevent the timely conversion of the shares of Series A Convertible Preferred Stock.
7.6 No Charge or Payment. The issuance of shares of Common Stock upon conversion of shares of Series A Convertible Preferred Stock pursuant to Section 7.2 shall be made without payment of additional consideration by, or other charge, cost, or tax to, the Holder in respect thereof.
8. Stock Dividends and Stock Splits. If the Corporation, at any time while this Series A Convertible Preferred Stock is outstanding: (A) pays a stock dividend or otherwise makes a distribution or distributions payable in shares of Common Stock (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Corporation upon conversion of this Series A Convertible Preferred Stock) with respect to the then outstanding shares of Common Stock; (B) subdivides outstanding shares of Common Stock into a larger number of shares; or (C) combines (including by way of a reverse stock split) outstanding shares of Common Stock into a smaller number of shares, then the Conversion Ratio shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock (excluding any treasury shares of the Corporation) outstanding immediately after such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately before such event (excluding any treasury shares of the Corporation). Any adjustment made pursuant to this Section 8 shall become effective immediately after the record date for the determination of stockholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision or combination.
9. Reissuance of Series A Convertible Preferred Stock. Any shares of Series A Convertible Preferred Stock converted, or otherwise acquired by the Corporation or any Subsidiary shall be cancelled and returned as authorized and unissued shares of capital stock of the Corporation.
10. Notices. Except as otherwise provided herein, all notices, requests, consents, claims, demands, waivers, and other communications hereunder shall be in writing and shall be deemed to have been given: (a) when delivered by hand (with written confirmation of receipt); (b) when received by the addressee if sent by a nationally recognized overnight courier (receipt requested); (c) on the date sent by facsimile or e-mail of a PDF document (with confirmation of transmission) if sent during normal business hours of the recipient, and on the next Business Day if sent after normal business hours of the recipient; or (d) on the third day after the date mailed, by certified or registered mail, return receipt requested, postage prepaid. Such communications must be sent (a) to the Corporation, at its principal executive offices and (b) to any stockholder, at such holder’s address at it appears in the stock records of the Corporation (or at such other address for a stockholder as shall be specified in a notice given in accordance with this Section 10).
11. Amendment and Waiver. No provision of this Certificate of Designation may be amended, modified, or waived except by an instrument in writing executed by the Corporation and the Holders of a majority of the outstanding shares of Series A Convertible Preferred Stock.
[signature page follows]
Annex E-3
IN WITNESS WHEREOF, Apimeds Pharmaceuticals US, Inc. has caused this Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock to be duly executed by its Chief Executive Officer on December 1, 2025.
|
APIMEDS PHARMACEUTICALS US, INC. |
||||
|
By: |
/s/ Erik Emerson |
|||
|
Name: |
Erik Emerson |
|||
|
Title: |
Chief Executive Officer |
|||
[Signature Page to Series A Preferred Stock Certificate of Designation]
Annex E-4
STATE OF DELAWARE
CERTIFICATE OF CORRECTION TO
CERTIFICATE OF DESIGNATION OF PREFERENCES,
RIGHTS AND LIMITATIONS OF
SERIES A CONVERTIBLE PREFERRED STOCK OF
APIMEDS PHARMACEUTICALS US, INC.
Apimeds Pharmaceuticals US, Inc. a corporation organized and existing under the General Corporation Law of the State of Delaware (the “Corporation”), hereby certifies as follows:
1. The name of the corporation is Apimeds Pharmaceuticals US, Inc.
2. That a Certificate of Designation of Preferences, Rights and Limitations of Series A Convertible Preferred Stock was filed with the Secretary of State of the State of Delaware on December 1, 2025 (the “Certificate of Designation”), and that the Certificate of Designation requires correction as permitted by subsection (f) of Section 103 of the General Corporation Law of the State of Delaware.
3. The inaccuracy or defect of said Certificate of Designation is as follows: Section 2 is inaccurate due to a scrivener’s error in the number of shares of common stock designated as “Series A Convertible Preferred Stock”.
4. Section 2 of the Certificate of Designation is hereby corrected to read in its entirety as follows:
“2. Designation. There shall be a series of Preferred Stock that shall be designated as “Series A Convertible Preferred Stock” (the “Series A Convertible Preferred Stock”) and the number of shares constituting such series shall be 7,477,017. The rights, preferences, powers, restrictions, and limitations of the Series A Convertible Preferred Stock shall be as set forth herein.”
[SIGNATURE PAGE FOLLOWS]
Annex F-1
IN WITNESS WHEREOF, the Corporation has caused this Certificate of Correction to be signed by the undersigned authorized officer this 10th day of December, 2025.
|
APIMEDS PHARMACEUTICALS US, INC. |
||||
|
By: |
/s/ Vin Menon |
|||
|
Name: |
Vin Menon |
|||
|
Title: |
Chief Executive Officer |
|||
Annex F-2
CERTIFICATE OF AMENDMENT
OF AMENDED AND RESTATED CERTIFICATE OF INCORPORATION
OF APIMEDS PHARMACEUTICALS US, INC.
Apimeds Pharmaceuticals US, Inc., a corporation organized and existing under the laws of the State of Delaware (the “Corporation”), does hereby certify as follows:
1. The name of the Corporation is Apimeds Pharmaceuticals US, Inc. The original certificate of incorporation of the Corporation was filed with the Secretary of State of the State of Delaware on May 11, 2020 (the “Certificate”). The Amended and Restated Certificate of Incorporation (the “Amended and Restated Certificate”) was filed with the Secretary of State of the State of Delaware on December 5, 2023, which both restated and amended the provisions of the Certificate.
2. The Amended and Restated Certificate of the Corporation is amended by replacing article FOURTH with the following:
“The Company is authorized to issue two classes of stock to be designated, respectively, “Common Stock” and “Preferred Stock.” The total number of shares which the Company is authorized to issue is 110,000,000 shares. 100,000,000 shares shall be Common Stock, each having a par value of $0.001. 10,000,000 shares shall be Preferred Stock, each having a par value of $0.001.
The Preferred Stock may be issued from time to time in one or more series. The Board of Directors of the Corporation is hereby expressly authorized to provide for the issue of any or all of the unissued and undesignated shares of the Preferred Stock in one or more series, and to fix the number of shares and to determine or alter for each such series, such voting powers, full or limited, or no voting powers, and such designation, preferences, and relative, participating, optional, or other rights and such qualifications, limitations, or restrictions thereof, as shall be stated and expressed in the resolution or resolutions adopted by the Board of Directors of the Corporation providing for the issuance of such shares and as may be permitted by the DGCL. The Board of Directors of the Corporation is also expressly authorized to increase or decrease the number of shares of any series subsequent to the issuance of shares of that series, but not below the number of shares of such series then outstanding. In case the number of shares of any series shall be decreased in accordance with the foregoing sentence, the shares constituting such decrease shall resume the status that they had prior to the adoption of the resolution originally fixing the number of shares of such series. The number of authorized shares of Preferred Stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of the stock of the Company entitled to vote thereon, without a separate vote of the holders of the Preferred Stock, or of any series thereof, unless a vote of any such holders is required pursuant to the terms of any certificate of designation filed with respect to any series of Preferred Stock.
Each outstanding share of Common Stock shall entitle the holder thereof to one vote on each matter properly submitted to the stockholders of the Company for their vote; provided, however, that, except as otherwise required by law, holders of Common Stock shall not be entitled to vote on any amendment to this Amended and Restated Certificate (including any certificate of designation filed with respect to any series of Preferred Stock) that relates solely to the terms of one or more outstanding series of Preferred Stock if the holders of such affected series of Preferred Stock are entitled, either separately or together as a class with the holders of one or more other such series of Preferred Stock, to vote thereon by law or pursuant to this Amended and Restated Certificate (including any certificate of designation filed with respect to any series of Preferred Stock).
Upon the filing and effectiveness (the “Effective Time”) of this amendment to the Corporation’s Amended and Restated Certificate, pursuant to the DGCL, each ten (10) shares of the Common Stock issued immediately prior to the Effective Time (the “Old Common Stock”) shall be reclassified and combined into one validly issued, fully paid and non-assessable share of the Corporation’s Common Stock, $0.001 par value per share (the “New Common Stock”), without any action by the holder thereof, subject to the treatment of fractional share interests as described below (the “Reverse Stock Split”). No fractional shares of New Common Stock shall be issued as a result of the Reverse Stock Split and, any
Annex G-1
person who would otherwise be entitled to a fractional share of New Common Stock as a result of the Reverse Stock Split, following the Effective Time, shall be entitled to receive a whole share of New Common Stock in lieu of any fractional share created as a result of such Reverse Stock Split. Each book entry position that theretofore represented shares of Old Common Stock shall thereafter represent that number of shares of New Common Stock into which the shares of Old Common Stock represented by such book entry position shall have been reclassified and combined.
The Reverse Stock Split shall not affect the total number of shares of capital stock, including the Common Stock, that the Corporation is authorized to issue, which shall remain as set forth under this article.”
3. This Certificate of Amendment has been duly adopted by the Board of Directors of the Corporation and stockholders of the Corporation in accordance with Section 242 of the General Corporation Law of the State of Delaware.
4. This Certificate of Amendment shall become effective immediately upon filing.
IN WITNESS WHEREOF, the Corporation has caused this Certificate of Amendment to be duly executed in its corporate name as of the [__]th day of [__], 2025.
|
APIMEDS PHARMACEUTICALS US, INC. |
||||
|
By: |
|
|||
|
Name: |
||||
|
Title: |
||||
Annex G-2
AMENDMENT TO
APIMEDS PHARMACEUTICALS US, INC. 2024
EQUITY INCENTIVE PLAN
This Amendment (this “Amendment”) to the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan, as amended (the “Plan”) is dated as of [ ], 2025 (“Effective Date”). Capitalized terms used in this Amendment and not otherwise defined herein shall have the meanings ascribed to such terms in the Plan.
WHEREAS, the Company currently maintaining the Plan;
WHEREAS, pursuant to Section 13 of the Plan, the Board may at any time amend, alter, suspend, or terminate the Plan; and
WHEREAS, the Board believes that it is in the best interests of the Company and its shareholders to amend the Plan to increase the shares subject to the Plan.
NOW, THEREFORE, the following amendments and modifications are hereby made a part of the Plan effective as of the date hereof:
1. Subject to the approval of the Company’s shareholders, effective as of the Effective Date, Section 5(b) of the Plan is hereby amended in its entirety to read as follows:
“Subject to Section 12 of the Plan, Awards granted under the Plan shall be subject to the following limitation: the Committee is authorized to deliver under the Plan an aggregate of 2,096,679 Common Shares.
2. Full Force and Effect. In all other respects, the Plan, as amended, is hereby ratified and confirmed and shall remain in full force and effect.
3. Construction. This Amendment shall be construed in accordance with the internal law of the State of Delaware.
IN WITNESS WHEREOF, the Company has executed this Amendment to the Plan as of the Effective Date.
|
APIMEDS PHARMACEUTICALS US, INC. |
||||
|
By: |
|
|||
|
Name: |
||||
|
Title: |
||||
Annex H-1
APIMEDS PHARMACEUTICALS US, INC.
2024 EQUITY INCENTIVE PLAN
1. Purpose. The purpose of the Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan (the “Plan”) is to provide a means through which the Company and its Affiliates may attract and retain key personnel and to provide a means whereby directors, officers, employees, consultants and advisors (and prospective directors, officers, employees, consultants and advisors) of the Company and its Affiliates can acquire and maintain an equity interest in the Company, or be paid incentive compensation, which may (but need not) be measured by reference to the value of Common Shares, thereby strengthening their commitment to the welfare of the Company and its Affiliates and aligning their interests with those of the Company’s shareholders.
2. Definitions. The following definitions shall be applicable throughout the Plan:
(a) “Affiliate” means, at the time of determination, (i) any person or entity that directly or indirectly controls, is controlled by or is under common control with the Company and/or (ii) to the extent provided by the Committee, any person or entity in which the Company has a significant interest. The term “control” (including, with correlative meaning, the terms “controlled by” and “under common control with”), as applied to any person or entity, means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such person or entity, whether through the ownership of voting or other securities, by contract or otherwise. The Committee will have the authority to determine the time or times at which “Affiliate” is determined within the forgoing definition.
(b) “Award” means, individually or collectively, any Incentive Stock Option, Non-Qualified Stock Option, Stock Appreciation Right, Restricted Stock, Restricted Stock Unit, Stock Bonus Award, and Performance Compensation Award granted under the Plan.
(c) “Board” means the Board of Directors of the Company.
(d) “Business Combination” has the meaning given such term in the definition of “Change in Control.”
(e) “Cause” shall have the meaning set forth in the applicable Participant’s employment or similar agreement with the Company or its Affiliates and in the absence of such agreement or definition, shall mean, (i) the Company or an Affiliate having “cause” to terminate a Participant’s employment or service, as defined in any employment or consulting or similar agreement between the Participant and the Company or an Affiliate in effect at the time of such termination or (ii) in the absence of any such employment or consulting or similar agreement (or the absence of any definition of “Cause” contained therein), (A) gross misconduct by the Participant which results in loss, damage or injury to the Company or any of its Affiliates, its goodwill, business or reputation; (B) the commission or attempted commission of an act of embezzlement, fraud or breach of fiduciary duty which results in loss, damage or injury to the Company or any of its Affiliates, its goodwill, business or reputation; (C) the unauthorized disclosure or misappropriation of any trade secret or confidential information of the Company, any of its Affiliate or any third party who has a business relationship with the Company; (D) the Participant’s conviction of or plea of nolo contendere to, a felony under any state or federal law; (E) the violation (or potential violation) by the Participant, in any material respect, of a non-competition, non-solicitation, non-disclosure or assignment of inventions covenant between the Participant and the Company or any of its Affiliates; (F) the Participant’s failure to perform the Participant’s assigned duties and responsibilities to the reasonable satisfaction of the Company which failure continues, in the reasonable judgment of the Company, after written notice given to the Participant by the Company; or (G) the use of controlled substances, illicit drugs, alcohol or other substances or behavior which interferes with the Participant’s ability to perform his or her services for the Company or any of its Affiliates or which otherwise results in loss, damage or injury to the Company, its goodwill, business or reputation. Any determination of whether Cause exists shall be made by the Committee in its sole discretion.
(f) “Change in Control” shall, in the case of a particular Award, unless the applicable Award agreement states otherwise or contains a different definition of “Change in Control,” be deemed to occur upon:
(i) Any sale, lease, exchange or other transfer (in one or a series of related transactions) of all or substantially all of the assets of the Company;
Annex I-1
(ii) Any “Person” as such term is used in Section 13(d) and Section 14(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) becomes, directly or indirectly, the “beneficial owner” as defined in Rule 13d-3 under the Exchange Act of securities of the Company that represent fifty percent (50%) or more of the combined voting power of the Company’s then outstanding voting securities (the “Outstanding Company Voting Securities”); provided, however, that for purposes of this Section 2(f)(ii), the following acquisitions shall not constitute a Change in Control: (I) any acquisition directly from the Company principally for bona fide equity financing purposes, (II) any acquisition by the Company, (III) any acquisition by any employee benefit plan (or related trust) sponsored or maintained by the Company or any Affiliate, (IV) any acquisition by any corporation pursuant to a transaction that complies with Sections 2(f)(iv)(A) and 2(f)(iv)(B), (V) any acquisition involving beneficial ownership of less than fifty percent (50%) of the then-outstanding Common Shares (the “Outstanding Company Common Shares”) or the Outstanding Company Voting Securities that is determined by the Board, based on review of public disclosure by the acquiring Person with respect to its passive investment intent, not to have a purpose or effect of changing or influencing the control of the Company; provided, however, that for purposes of this clause (V), any such acquisition in connection with (x) an actual or threatened election contest with respect to the election or removal of directors or other actual or threatened solicitation of proxies or consents or (y) any “Business Combination” (as defined below) shall be presumed to be for the purpose or with the effect of changing or influencing the control of the Company;
(iii) During any period of not more than two (2) consecutive years, individuals who constitute the Board as of the beginning of the period (the “Incumbent Directors”) cease for any reason to constitute at least a majority of the Board, provided that any person becoming a director subsequent to the beginning of such period, whose election or nomination for election was approved by a vote of at least two-thirds (2/3) of the Incumbent Directors then on the Board (either by a specific vote or by approval of the proxy statement of the Company in which such person is named as a nominee for director, without written objection to such nomination) will be an Incumbent Director; provided, however, that no individual initially elected or nominated as a director of the Company as a result of an actual or threatened election contest with respect to directors or as a result of any other actual or threatened solicitation of proxies by or on behalf of any person other than the Board will be deemed to be an Incumbent Director;
(iv) Consummation of a merger, amalgamation or consolidation (a “Business Combination”) of the Company with any other corporation, unless, following such Business Combination, (A) all or substantially all of the individuals and entities that were the beneficial owners of the Outstanding Company Common Shares and the Outstanding Company Voting Securities immediately prior to such Business Combination beneficially own, directly or indirectly, more than fifty percent (50%) of the then-outstanding shares of common stock (or, for a non-corporate entity, equivalent securities) and the combined voting power of the then-outstanding voting securities entitled to vote generally in the election of directors (or, for a non-corporate entity, equivalent governing body), as the case may be, of the entity resulting from such Business Combination (including, without limitation, an entity that, as a result of such transaction, owns the Company or all or substantially all of the Company’s assets either directly or through one or more subsidiaries) in substantially the same proportions as their ownership immediately prior to such Business Combination of the Outstanding Company Common Shares and the Outstanding Company Voting Securities, as the case may be, and (B) at least a majority of the members of the board of directors (or, for a non-corporate entity, equivalent governing body) of the entity resulting from such Business Combination were Incumbent Directors at the time of the execution of the initial agreement or of the action of the Board providing for such Business Combination;
(v) Shareholder approval of a plan of complete liquidation of the Company.
(g) “Code” means the Internal Revenue Code of 1986, as amended, and any successor thereto. Reference in the Plan to any section of the Code shall be deemed to include any regulations or other interpretative guidance under such section, and any amendments or successor provisions to such section, regulations or guidance.
(h) “Committee” means a committee of at least two (2) people as the Board may appoint to administer the Plan or, if no such committee has been appointed by the Board, the Board.
(i) “Common Shares” means the shares of the Company’s common stock, par value $0.01 per share.
(j) “Company” means Apimeds Pharmaceuticals US, Inc., a Delaware corporation.
(k) “Date of Grant” means the date on which the granting of an Award is authorized, or such other date as may be specified in such authorization.
Annex I-2
(l) “Effective Date” means the date on which the Plan is approved by the shareholders of the Company.
(m) “Eligible Director” means a person who is a “non-employee director” within the meaning of Rule 16b-3 under the Exchange Act.
(n) “Eligible Person” with respect to an Award denominated in Common Shares, means any (i) individual employed by the Company or an Affiliate; (ii) director of the Company or an Affiliate; (iii) consultant or advisor to the Company or an Affiliate; provided that if the Securities Act applies such persons must be eligible to be offered securities registrable on Form S-8 under the Securities Act; or (iv) prospective employees, directors, officers, consultants or advisors who have accepted offers of employment or consultancy from the Company or its Affiliates (and would satisfy the provisions of clauses (i) through (iii) above once he or she begins employment with or begins providing services to the Company or its Affiliates).
(o) “Exchange Act” has the meaning given such term in the definition of “Change in Control,” and any reference in the Plan to any section of (or rule promulgated under) the Exchange Act shall be deemed to include any rules, regulations or other interpretative guidance under such section or rule, and any amendments or successor provisions to such section, rules, regulations or guidance.
(p) “Exercise Price” has the meaning given such term in Section 7(b) of the Plan.
(q) “Fair Market Value” means, as of any date, the value of Common Shares determined as follows:
(i) If the Common Shares are listed on any established stock exchange or a national market system will be the closing sales price for such shares (or the closing bid, if no sales were reported) as quoted on such exchange or system on the day of determination, as reported in The Wall Street Journal or such other source as the Committee deems reliable;
(ii) If the Common Shares are regularly quoted by a recognized securities dealer but selling prices are not reported, the Fair Market Value of a Common Share will be the mean between the high bid and low asked prices for the Common Shares on the day of determination, as reported in The Wall Street Journal or such other source as the Committee deems reliable; or
(iii) In the absence of an established market for the Common Shares, the Fair Market Value will be determined in good faith by the Committee.
(r) “Good Reason” shall have the meaning set forth in the applicable Participant’s employment or similar agreement with the Company or its Affiliates and in the absence of such agreement or definition, shall mean that one or more of the following has occurred without the Participant’s written consent:
(i) a material negative change in the nature or scope of the Participant’s responsibilities, duties or authority;
(ii) a material reduction in the Participant’s base compensation, excluding any reduction up to ten percent (10%) that is applied to all similarly situated service providers of the Company; or
(iii) the Participant’s required re-location to a worksite location which is more than fifty (50) miles from the Participant’s then current principal worksite without the Participant’s prior written consent (such consent not to be unreasonably withheld).
provided that, in any such case, the Participant provides written notice to the Company that the event giving rise to such claim of Good Reason has occurred within thirty (30) days after the occurrence of such event, and such Good Reason remains uncured thirty (30) days after the Participant has provided such written notice; provided further that any resignation of the Participant’s employment or service for “Good Reason” occurs no later than thirty (30) days following the expiration of such cure period.
(s) “Immediate Family Members” shall have the meaning set forth in Section 15(b).
(t) “Incentive Stock Option” means an Option that is designated by the Committee as an incentive stock option as described in Section 422 of the Code and otherwise meets the requirements set forth in the Plan.
Annex I-3
(u) “Indemnifiable Person” shall have the meaning set forth in Section 4(e) of the Plan.
(v) “Mature Shares” means Common Shares owned by a Participant that are not subject to any pledge or security interest and that have been either previously acquired by the Participant on the open market or meet such other requirements, if any, as the Committee may determine are necessary in order to avoid an accounting earnings charge on account of the use of such shares to pay the Exercise Price or satisfy a tax or deduction obligation of the Participant.
(w) “Non-Qualified Stock Option” means an Option that is not designated by the Committee as an Incentive Stock Option.
(x) “Option” means an Award granted under Section 7 of the Plan.
(y) “Option Period” has the meaning given such term in Section 7(c) of the Plan.
(z) “Outstanding Company Common Shares” has the meaning given such term in the definition of “Change in Control.”
(aa) “Outstanding Company Voting Securities” has the meaning given such term in the definition of “Change in Control.”
(bb) “Participant” means an Eligible Person who has been selected by the Committee to participate in the Plan and to receive an Award pursuant to Section 6 of the Plan.
(cc) “Performance Compensation Award” shall mean any Award designated by the Committee as a Performance Compensation Award pursuant to Section 11 of the Plan.
(dd) “Performance Criteria” shall mean the criterion or criteria that the Committee shall select for purposes of establishing the Performance Goal(s) for a Performance Period with respect to any Performance Compensation Award under the Plan.
(ee) “Performance Formula” shall mean, for a Performance Period, the one or more formulae applied against the relevant Performance Goal to determine, with regard to the Performance Compensation Award of a particular Participant, whether all, some portion but less than all, or none of the Performance Compensation Award has been earned for the Performance Period.
(ff) “Performance Goals” shall mean, for a Performance Period, the one or more goals established by the Committee for the Performance Period based upon the Performance Criteria.
(gg) “Performance Period” shall mean the one or more periods of time, as the Committee may select, over which the attainment of one or more Performance Goals will be measured for the purpose of determining a Participant’s right to, and the payment of, a Performance Compensation Award.
(hh) “Permitted Transferee” shall have the meaning set forth in Section 15(b) of the Plan.
(ii) “Person” has the meaning given such term in the definition of “Change in Control.”
(jj) “Plan” means this Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan, as amended from time to time.
(kk) “Qualifying Termination” means, except as otherwise provided by the Committee as set forth in the Award, the occurrence of either a termination of a Participant’s employment by the Company without Cause or for Good Reason, in either case, occurring on or within the twelve (12) month period (or such other period specified in the applicable Award Agreement) following the consummation of a Change in Control.
(ll) “Restricted Period” means the period of time determined by the Committee during which an Award is subject to restrictions or, as applicable, the period of time within which performance is measured for purposes of determining whether an Award has been earned.
Annex I-4
(mm) “Restricted Stock Unit” means an unfunded and unsecured promise to deliver Common Shares, cash, other securities or other property, subject to certain performance or time-based restrictions (including, without limitation, a requirement that the Participant remain continuously employed or provide continuous services for a specified period of time), granted under Section 9 of the Plan.
(nn) “Restricted Stock” means Common Shares, subject to certain specified performance or time-based restrictions (including, without limitation, a requirement that the Participant remain continuously employed or provide continuous services for a specified period of time), granted under Section 9 of the Plan.
(oo) “Retirement” means, in the case of a particular Award, the definition set forth in the applicable Award Agreement.
(pp) “SAR Period” has the meaning given such term in Section 8(b) of the Plan.
(qq) “Securities Act” means the Securities Act of 1933, as amended, and any successor thereto. Reference in the Plan to any section of the Securities Act shall be deemed to include any rules, regulations or other interpretative guidance under such section, and any amendments or successor provisions to such section, rules, regulations or guidance.
(rr) “Stock Appreciation Right” or “SAR” means an Award granted under Section 8 of the Plan.
(ss) “Stock Bonus Award” means an Award granted under Section 10 of the Plan.
(tt) “Strike Price” means, except as otherwise provided by the Committee in the case of Substitute Awards, (i) in the case of a SAR granted in tandem with an Option, the Exercise Price of the related Option, or (ii) in the case of a SAR granted independent of an Option, the Fair Market Value on the Date of Grant.
(uu) “Subsidiary” means, with respect to any specified Person:
(i) any corporation, association or other business entity of which more than fifty percent (50%) of the total voting power of shares (without regard to the occurrence of any contingency and after giving effect to any voting agreement or shareholders’ agreement that effectively transfers voting power) is at the time owned or controlled, directly or indirectly, by that Person or one or more of the other Subsidiaries of that Person (or a combination thereof); and
(ii) any partnership (or any comparable foreign entity (a) the sole general partner (or functional equivalent thereof) or the managing general partner of which is such Person or Subsidiary of such Person or (b) the only general partners (or functional equivalents thereof) of which are that Person or one or more Subsidiaries of that Person (or any combination thereof).
(vv) “Substitute Award” has the meaning given such term in Section 5(e).
3. Effective Date; Duration. The Plan shall be effective as of the Effective Date. The expiration date of the Plan, on and after which date no Awards may be granted hereunder, shall be the tenth anniversary of the Effective Date; provided, however, that such expiration shall not affect Awards then outstanding, and the terms and conditions of the Plan shall continue to apply to such Awards.
4. Administration.
(a) The Committee shall administer the Plan. To the extent required to comply with the applicable provisions of Rule 16b-3 promulgated under the Exchange Act (if the Board is not acting as the Committee under the Plan), it is intended that each member of the Committee shall, at the time he or she takes any action with respect to an Award under the Plan, be an Eligible Director. However, the fact that a Committee member shall fail to qualify as an Eligible Director shall not invalidate any Award granted by the Committee that is otherwise validly granted under the Plan.
(b) Subject to the provisions of the Plan and applicable law, the Committee shall have the sole and plenary authority, in addition to other express powers and authorizations conferred on the Committee by the Plan or by the Board, to: (i) designate Participants; (ii) determine the type or types of Awards to be granted to a Participant; (iii) determine the number of Common Shares to be covered by, or with respect to which payments, rights, or other matters are to be calculated in connection with, Awards; (iv) determine the form of Award agreement and the terms
Annex I-5
and conditions of any Award; (v) determine whether, to what extent, and under what circumstances Awards may be settled or exercised in cash, Common Shares, other securities, other Awards or other property, or canceled, forfeited, or suspended and the method or methods by which Awards may be settled, exercised, canceled, forfeited, or suspended; (vi) determine whether, to what extent, and under what circumstances the delivery of cash, Common Shares, other securities, other Awards or other property and other amounts payable with respect to an Award shall be deferred either automatically or at the election of the Participant or of the Committee; (vii) interpret, administer, reconcile any inconsistency in, correct any defect in and/or supply any omission in the Plan and any instrument or agreement relating to, or Award granted under, the Plan; (viii) establish, amend, suspend, or waive any rules and regulations and appoint such agents as the Committee shall deem appropriate for the proper administration of the Plan; (ix) accelerate the vesting or exercisability of, payment for or lapse of restrictions on, Awards, including, but not limited to, upon a Qualifying Termination; and (x) make any other determination and take any other action that the Committee deems necessary or desirable for the administration of the Plan.
(c) The Committee may delegate to one (1) or more officers of the Company or any Affiliate the authority to act on behalf of the Committee with respect to any matter, right, obligation, or election that is the responsibility of or that is allocated to the Committee herein, and that may be so delegated as a matter of law, except for grants of Awards to persons subject to Section 16 of the Exchange Act.
(d) Unless otherwise expressly provided in the Plan, all designations, determinations, interpretations, and other decisions under or with respect to the Plan or any Award or any documents evidencing Awards granted pursuant to the Plan shall be within the sole discretion of the Committee, may be made at any time and shall be final, conclusive and binding upon all persons or entities, including, without limitation, the Company, any Affiliate, any Participant, any holder or beneficiary of any Award, and any shareholder of the Company.
(e) No member of the Board, the Committee, delegate of the Committee or any employee or agent of the Company (each such person, an “Indemnifiable Person”) shall be liable for any action taken or omitted to be taken or any determination made in good faith with respect to the Plan or any Award hereunder. Each Indemnifiable Person shall be indemnified and held harmless by the Company against and from any loss, cost, liability, or expense (including attorneys’ fees) that may be imposed upon or incurred by such Indemnifiable Person in connection with or resulting from any action, suit or proceeding to which such Indemnifiable Person may be a party or in which such Indemnifiable Person may be involved by reason of any action taken or omitted to be taken under the Plan or any Award agreement and against and from any and all amounts paid by such Indemnifiable Person with the Company’s approval, in settlement thereof, or paid by such Indemnifiable Person in satisfaction of any judgment in any such action, suit or proceeding against such Indemnifiable Person, provided that the Company shall have the right, at its own expense, to assume and defend any such action, suit or proceeding and once the Company gives notice of its intent to assume the defense, the Company shall have sole control over such defense with counsel of the Company’s choice. The foregoing right of indemnification shall not be available to an Indemnifiable Person to the extent that a final judgment or other final adjudication (in either case not subject to further appeal) binding upon such Indemnifiable Person determines that the acts or omissions of such Indemnifiable Person giving rise to the indemnification claim resulted from such Indemnifiable Person’s bad faith, fraud or willful criminal act or omission or that such right of indemnification is otherwise prohibited by law or by the Company’s Articles of Incorporation or Bylaws. The foregoing right of indemnification shall not be exclusive of any other rights of indemnification to which such Indemnifiable Persons may be entitled under the Company’s Articles of Incorporation or Bylaws, as a matter of law, or otherwise, or any other power that the Company may have to indemnify such Indemnifiable Persons or hold them harmless.
(f) Notwithstanding anything to the contrary contained in the Plan, the Board may, in its sole discretion, at any time and from time to time, grant Awards and administer the Plan with respect to such Awards. In any such case, the Board shall have all the authority granted to the Committee under the Plan.
5. Grant of Awards; Shares Subject to the Plan; Limitations.
(a) The Committee may, from time to time, grant Options, Stock Appreciation Rights, Restricted Stock, Restricted Stock Units, Stock Bonus Awards and/or Performance Compensation Awards to one or more Eligible Persons.
(b) Subject to Section 12 of the Plan, Awards granted under the Plan shall be subject to the following limitation: the Committee is authorized to deliver under the Plan an aggregate of Four Million (4,000,000) Common Shares.
Annex I-6
(c) In the event that (i) any Option or other Award granted hereunder is exercised through the tendering of Common Shares (either actually or by attestation) or by the withholding of Common Shares by the Company, or (ii) tax or deduction liabilities arising from such Option or other Award are satisfied by the tendering of Common Shares (either actually or by attestation) or by the withholding of Common Shares by the Company, then in each such case the Common Shares so tendered or withheld shall be added to the Common Shares available for grant under the Plan on a one-for-one basis. Shares underlying Awards under the Plan that are forfeited, cancelled, expire unexercised, or are settled in cash are available again for Awards under the Plan.
(d) Common Shares delivered by the Company in settlement of Awards may be authorized and unissued shares, shares held in the treasury of the Company, shares purchased on the open market or by private purchase, or a combination of the foregoing.
(e) Awards may, in the sole discretion of the Committee, be granted under the Plan in assumption of, or in substitution for, outstanding awards previously granted by an entity acquired by the Company or with which the Company combines (“Substitute Awards”). The number of Common Shares underlying any Substitute Awards shall not be counted against the aggregate number of Common Shares available for Awards under the Plan.
6. Eligibility. Participation shall be limited to Eligible Persons who have entered into an Award agreement or who have received written notification from the Committee, or from a person designated by the Committee, that they have been selected to participate in the Plan.
7. Options.
(a) Generally. Each Option granted under the Plan shall be evidenced by an Award agreement (whether in paper or electronic medium (including email or the posting on a web site maintained by the Company or a third party under contract with the Company)). Each Option so granted shall be subject to the conditions set forth in this Section 7, and to such other conditions not inconsistent with the Plan as may be reflected in the applicable Award agreement. All Options granted under the Plan shall be Non-Qualified Stock Options unless the applicable Award agreement expressly states that the Option is intended to be an Incentive Stock Option. The maximum aggregate number of Common Shares that may be issued through the exercise of Incentive Stock Options granted under the Plan is Four Million (4,000,000) Common Shares. Incentive Stock Options shall be granted only to Eligible Persons who are employees of the Company and its Affiliates, and no Incentive Stock Option shall be granted to any Eligible Person who is ineligible to receive an Incentive Stock Option under the Code. No Option shall be treated as an Incentive Stock Option unless the Plan has been approved by the shareholders of the Company in a manner intended to comply with the stockholder approval requirements of Section 422(b)(1) of the Code; provided that any Option intended to be an Incentive Stock Option shall not fail to be effective solely on account of a failure to obtain such approval, but rather such Option shall be treated as a Non-Qualified Stock Option unless and until such approval is obtained. In the case of an Incentive Stock Option, the terms and conditions of such grant shall be subject to and comply with such rules as may be prescribed by Section 422 of the Code. If for any reason an Option intended to be an Incentive Stock Option (or any portion thereof) shall not qualify as an Incentive Stock Option, then, to the extent of such non-qualification, such Option or portion thereof shall be regarded as a Non-Qualified Stock Option appropriately granted under the Plan.
(b) Exercise Price. Except with respect to Substitute Awards, the exercise price (“Exercise Price”) per Common Share for each Option shall not be less than one hundred percent (100%) of the Fair Market Value of such share determined as of the Date of Grant; provided, however, that in the case of an Incentive Stock Option granted to an employee who, at the time of the grant of such Option, owns shares representing more than ten percent (10%) of the total combined voting power of all classes of shares of the Company or any related corporation (as determined in accordance with Treasury Regulation Section 1.422-2(f)), the Exercise Price per share shall not be less than one hundred and ten percent (110%) of the Fair Market Value per share on the Date of Grant and provided further, that, notwithstanding any provision herein to the contrary, the Exercise Price shall not be less than the par value per Common Share.
(c) Vesting and Expiration. Options shall vest and become exercisable in such manner and on such date or dates determined by the Committee and shall expire after such period, not to exceed ten (10) years, as may be determined by the Committee (the “Option Period”); provided, however, that the Option Period shall not exceed five (5) years from the Date of Grant in the case of an Incentive Stock Option granted to a Participant who on the Date of Grant owns shares representing more than ten percent (10%) of the total combined voting power of all classes of shares of the Company or any related corporation (as determined in accordance with Treasury
Annex I-7
Regulation Section 1.422-2(f)); provided, further, that notwithstanding any vesting dates set by the Committee, the Committee may, in its sole discretion, accelerate the exercisability of any Option, which acceleration shall not affect the terms and conditions of such Option other than with respect to exercisability. Unless otherwise provided by the Committee in an Award agreement: (i) the unvested portion of an Option shall expire upon the Participant’s termination of employment or service with the Company and its Affiliates, and the vested portion of such Option shall remain exercisable for (A) one (1) year following termination of employment or service by reason of such Participant’s death or disability (as determined by the Committee), but not later than the expiration of the Option Period or (B) ninety (90) days following termination of employment or service for any reason other than such Participant’s death or disability, and other than such Participant’s termination of employment or service for Cause, but not later than the expiration of the Option Period; and (ii) both the unvested and the vested portion of an Option shall expire upon the termination of the Participant’s employment or service by the Company for Cause. If the Option would expire at a time when the exercise of the Option would violate applicable securities laws, the expiration date applicable to the Option will be automatically extended to a date that is thirty (30) calendar days following the date such exercise would no longer violate applicable securities laws (so long as such extension shall not violate Section 409A of the Code); provided, that in no event shall such expiration date be extended beyond the expiration of the Option Period.
(d) Method of Exercise and Form of Payment. No Common Shares shall be delivered pursuant to any exercise of an Option until payment in full of the Exercise Price therefor is received by the Company and the Participant has paid to the Company an amount equal to any taxes required to be withheld or paid. Options that have become exercisable may be exercised by delivery of written or electronic notice of exercise to the Company in accordance with the terms of the Option accompanied by payment of the Exercise Price. The Exercise Price shall be payable (i) in cash, check, cash equivalent and/or Common Shares valued at the fair market value at the time the Option is exercised (including, pursuant to procedures approved by the Committee, by means of attestation of ownership of a sufficient number of Common Shares in lieu of actual delivery of such shares to the Company); provided that such Common Shares are not subject to any pledge or other security interest and are Mature Shares and; (ii) by such other method as the Committee may permit in accordance with applicable law, in its sole discretion, on a case by case basis, including without limitation: (A) in other property having a fair market value on the date of exercise equal to the Exercise Price or (B) if there is a public market for the Common Shares at such time, by means of a broker-assisted “cashless exercise” pursuant to which the Company is delivered a copy of irrevocable instructions to a stockbroker to sell the Common Shares otherwise deliverable upon the exercise of the Option and to deliver promptly to the Company an amount equal to the Exercise Price or (C) by a “net exercise” method whereby the Company withholds from the delivery of the Common Shares for which the Option was exercised that number of Common Shares having a fair market value equal to the aggregate Exercise Price for the Common Shares for which the Option was exercised. Fractional Common Shares may be issued or delivered pursuant to the Plan or any Award in the sole discretion of the Committee, and in the event the Committee determines that no fractional shares may be issued or delivered, the Committee shall determine whether cash, other securities or other property shall be paid or transferred in lieu of any fractional Common Shares, or whether such fractional Common Shares or any rights thereto shall be canceled, terminated or otherwise eliminated.
(e) Notification upon Disqualifying Disposition of an Incentive Stock Option. Each Participant awarded an Incentive Stock Option under the Plan shall notify the Company in writing immediately after the date he makes a disqualifying disposition of any Common Shares acquired pursuant to the exercise of such Incentive Stock Option. A disqualifying disposition is any disposition (including, without limitation, any sale) of such Common Shares before the later of (A) two (2) years after the Date of Grant of the Incentive Stock Option or (B) one (1) year after the date of exercise of the Incentive Stock Option. The Company may, if determined by the Committee and in accordance with procedures established by the Committee, retain possession of any Common Shares acquired pursuant to the exercise of an Incentive Stock Option as agent for the applicable Participant until the end of the period described in the preceding sentence.
(f) Compliance with Laws, etc. Notwithstanding the foregoing, in no event shall a Participant be permitted to exercise an Option in a manner that the Committee determines would violate the Sarbanes-Oxley Act of 2002, if applicable, or any other applicable law or the applicable rules and regulations of the Securities and Exchange Commission or the applicable rules and regulations of any securities exchange or inter-dealer quotation system on which the securities of the Company are listed or traded.
Annex I-8
8. Stock Appreciation Rights.
(a) Generally. Each SAR granted under the Plan shall be evidenced by an Award agreement (whether in paper or electronic medium (including email or the posting on a web site maintained by the Company or a third party under contract with the Company)). Each SAR so granted shall be subject to the conditions set forth in this Section 8, and to such other conditions not inconsistent with the Plan as may be reflected in the applicable Award agreement. Any Option granted under the Plan may include tandem SARs. The Committee also may award SARs to Eligible Persons independent of any Option.
(b) Exercise Price. The Exercise Price per Common Share for each SAR shall not be less than one hundred percent (100%) of the Fair Market Value of such share determined as of the Date of Grant.
(c) Vesting and Expiration. A SAR granted in connection with an Option shall become exercisable and shall expire according to the same vesting schedule and expiration provisions as the corresponding Option. A SAR granted independent of an Option shall vest and become exercisable and shall expire in such manner and on such date or dates determined by the Committee and shall expire after such period, not to exceed ten years, as may be determined by the Committee (the “SAR Period”); provided, however, that notwithstanding any vesting dates set by the Committee, the Committee may, in its sole discretion, accelerate the exercisability of any SAR, which acceleration shall not affect the terms and conditions of such SAR other than with respect to exercisability. Unless otherwise provided by the Committee in an Award agreement: (i) the unvested portion of a SAR shall expire upon termination of employment or service of the Participant granted the SAR, and the vested portion of such SAR shall remain exercisable for (A) one (1) year following termination of employment or service by reason of such Participant’s death or disability (as determined by the Committee), but not later than the expiration of the SAR Period or (B) ninety (90) days following termination of employment or service for any reason other than such Participant’s death or disability, and other than such Participant’s termination of employment or service for Cause, but not later than the expiration of the SAR Period; and (ii) both the unvested and the vested portion of a SAR shall expire upon the termination of the Participant’s employment or service by the Company for Cause. If the SAR would expire at a time when the exercise of the SAR would violate applicable securities laws, the expiration date applicable to the SAR will be automatically extended to a date that is thirty (30) calendar days following the date such exercise would no longer violate applicable securities laws (so long as such extension shall not violate Section 409A of the Code); provided, that in no event shall such expiration date be extended beyond the expiration of the SAR Period.
(d) Method of Exercise. SARs that have become exercisable may be exercised by delivery of written or electronic notice of exercise to the Company in accordance with the terms of the Award, specifying the number of SARs to be exercised and the date on which such SARs were awarded. Notwithstanding the foregoing, if on the last day of the Option Period (or in the case of a SAR independent of an option, the SAR Period), the fair market value exceeds the Strike Price, the Participant has not exercised the SAR or the corresponding Option (if applicable), and neither the SAR nor the corresponding Option (if applicable) has expired, such SAR shall be deemed to have been exercised by the Participant on such last day and the Company shall make the appropriate payment therefor.
(e) Payment. Upon the exercise of a SAR, the Company shall pay to the Participant an amount equal to the number of shares subject to the SAR that are being exercised multiplied by the excess, if any, of the fair market value of one Common Share on the exercise date over the Strike Price, less an amount equal to any taxes required to be withheld or paid. The Company shall pay such amount in cash, in Common Shares valued at fair market value, or any combination thereof, as determined by the Committee. Fractional Common Shares may be issued or delivered pursuant to the Plan or any Award in the sole discretion of the Committee, and in the event the Committee determines that no fractional shares may be issued or delivered, the Committee shall determine whether cash, other securities or other property shall be paid or transferred in lieu of any fractional Common Shares, or whether such fractional Common Shares or any rights thereto shall be canceled, terminated or otherwise eliminated.
9. Restricted Stock and Restricted Stock Units.
(a) Generally. Each grant of Restricted Stock and Restricted Stock Units shall be evidenced by an Award agreement (whether in paper or electronic medium (including email or the posting on a web site maintained by the Company or a third party under contract with the Company)). Each such grant shall be subject to the conditions set forth in this Section 9, and to such other conditions not inconsistent with the Plan as may be reflected in the applicable Award agreement.
Annex I-9
(b) Restricted Accounts; Escrow or Similar Arrangement. Upon the grant of Restricted Stock, a book entry in a restricted account shall be established in the Participant’s name at the Company’s transfer agent and, if the Committee determines that the Restricted Stock shall be held by the Company or in escrow rather than held in such restricted account pending the release of the applicable restrictions, the Committee may require the Participant to additionally execute and deliver to the Company (i) an escrow agreement satisfactory to the Committee, if applicable, and (ii) the appropriate share power (endorsed in blank) with respect to the Restricted Stock covered by such agreement. If a Participant shall fail to execute an agreement evidencing an Award of Restricted Stock and, if applicable, an escrow agreement and blank share power within the amount of time specified by the Committee, the Award shall be null and void. Subject to the restrictions set forth in this Section 9 and the applicable Award agreement, the Participant generally shall have the rights and privileges of a shareholder as to such Restricted Stock, including without limitation the right to vote such Restricted Stock and the right to receive dividends, if applicable. To the extent shares of Restricted Stock are forfeited, any share certificates issued to the Participant evidencing such shares shall be returned to the Company, and all rights of the Participant to such shares and as a shareholder with respect thereto shall terminate without further obligation on the part of the Company.
(c) Vesting; Acceleration of Lapse of Restrictions. Unless otherwise provided by the Committee in an Award agreement the unvested portion of Restricted Stock and Restricted Stock Units shall terminate and be forfeited upon termination of employment or service of the Participant granted the applicable Award.
(d) Delivery of Restricted Stock and Settlement of Restricted Stock Units.
(i) Upon the expiration of the Restricted Period with respect to any shares of Restricted Stock, the restrictions set forth in the applicable Award agreement shall be of no further force or effect with respect to such shares, except as set forth in the applicable Award agreement. If an escrow arrangement is used, upon such expiration, the Company shall deliver to the Participant, or his beneficiary, without charge, the share certificate evidencing the shares of Restricted Stock that have not then been forfeited and with respect to which the Restricted Period has expired (rounded down to the nearest full share). Dividends, if any, that may have been withheld by the Committee and attributable to any particular share of Restricted Stock shall be distributed to the Committee and attributable to any particular share of Restricted Stock shall be distributed to the Participant in cash or, at the sole discretion of the Committee, in Common Shares having a fair market value equal to the amount of such dividends, upon the release of restrictions on such share and, if such share is forfeited, the Participant shall have no right to such dividends (except as otherwise set forth by the Committee in the applicable Award agreement).
(ii) Unless otherwise provided by the Committee in an Award agreement, upon the expiration of the Restricted Period with respect to any outstanding Restricted Stock Units, the Company shall deliver to the Participant, or his beneficiary, without charge, one (1) Common Share for each such outstanding Restricted Stock Unit; provided, however, that the Committee may, in its sole discretion, elect to (i) pay cash or part cash and part Common Share in lieu of delivering only Common Shares in respect of such Restricted Stock Units or (ii) defer the delivery of Common Shares (or cash or part Common Shares and part cash, as the case may be) beyond the expiration of the Restricted Period if such delivery would result in a violation of applicable law until such time as is no longer the case. If a cash payment is made in lieu of delivering Common Shares, the amount of such payment shall be equal to the fair market value of the Common Shares as of the date on which the Restricted Period lapsed with respect to such Restricted Stock Units, less an amount equal to any taxes required to be withheld or paid.
10. Stock Bonus Awards. The Committee may issue unrestricted Common Shares, or other Awards denominated in Common Shares, under the Plan to Eligible Persons, either alone or in tandem with other awards, in such amounts as the Committee shall from time to time in its sole discretion determine. Each Stock Bonus Award granted under the Plan shall be evidenced by an Award agreement (whether in paper or electronic medium (including email or the posting on a web site maintained by the Company or a third party under contract with the Company)). Each Stock Bonus Award so granted shall be subject to such conditions not inconsistent with the Plan as may be reflected in the applicable Award agreement.
11. Performance Compensation Awards.
(a) Generally. The Committee shall have the authority, at the time of grant of any Award described in Sections 7 through 10 of the Plan, to designate such Award as a Performance Compensation Award. The Committee shall have the authority to make an award of a cash bonus to any Participant and designate such Award as a Performance Compensation Award. Unless otherwise determined by the Committee, all Performance Compensation Awards shall be evidenced by an Award agreement.
Annex I-10
(b) Discretion of Committee with Respect to Performance Compensation Awards. The Committee shall have the discretion to establish the terms, conditions and restrictions of any Performance Compensation Award. With regard to a particular Performance Period, the Committee shall have sole discretion to select the length of such Performance Period, the type(s) of Performance Compensation Awards to be issued, the Performance Criteria that will be used to establish the Performance Goal (s), the kind(s) and/or level(s) of the Performance Goals(s) that is (are) to apply and the Performance Formula.
(c) Performance Criteria. The Committee may establish Performance Criteria that will be used to establish the Performance Goal(s) for Performance Compensation Awards which may be based on the attainment of specific levels of performance of the Company (and/or one or more Affiliates, divisions, business segments or operational units, or any combination of the foregoing) and may include, without limitation, any of the following: (i) net earnings or net income (before or after taxes); (ii) basic or diluted earnings per share (before or after taxes); (iii) revenue or revenue growth (measured on a net or gross basis); (iv) gross profit or gross profit growth; (v) operating profit (before or after taxes); (vi) return measures (including, but not limited to, return on assets, capital, invested capital, equity, or sales); (vii) cash flow (including, but not limited to, operating cash flow, free cash flow, net cash provided by operations and cash flow return on capital); (viii) financing and other capital raising transactions (including, but not limited to, sales of the Company’s equity or debt securities); (ix) earnings before or after taxes, interest, depreciation and/or amortization; (x) gross or operating margins; (xi) productivity ratios; (xii) share price (including, but not limited to, growth measures and total shareholder return); (xiii) expense targets; (xiv) margins; (xv) productivity and operating efficiencies; (xvi) customer satisfaction; (xvii) customer growth; (xviii) working capital targets; (xix) measures of economic value added; (xx) inventory control; (xxi) enterprise value; (xxii) sales; (xxiii) debt levels and net debt; (xxiv) combined ratio; (xxv) timely launch of new facilities; (xxvi) client retention; (xxvii) employee retention; (xxviii) timely completion of rollouts of new products and services; (xxix) cost targets; (xxx) reductions and savings; (xxxi) productivity and efficiencies; (xxxii) strategic partnerships or transactions; and (xxxiii) personal targets, goals or completion of projects. Any one (1) or more of the Performance Criteria may be used on an absolute or relative basis to measure the performance of the Company and/or one or more Affiliates as a whole or any business unit(s) of the Company and/or one or more Affiliates or any combination thereof, as the Committee may deem appropriate, or any of the above Performance Criteria may be compared to the performance of a selected group of comparison or peer companies, or a published or special index that the Committee, in its sole discretion, deems appropriate, or as compared to various stock market indices. The Committee also has the authority to provide for accelerated vesting of any Award based on the achievement of Performance Goals pursuant to the Performance Criteria specified in this paragraph. Any Performance Criteria that are financial metrics, may be determined in accordance with United States Generally Accepted Accounting Principles (“GAAP”) or may be adjusted when established to include or exclude any items otherwise includable or excludable under GAAP.
(d) Modification of Performance Goal(s). The Committee is authorized at any time to adjust or modify the calculation of a Performance Goal for such Performance Period, based on and in order to appropriately reflect any specified circumstance or event that occurs during a Performance Period, including but not limited to the following: (i) asset write-downs; (ii) litigation or claim judgments or settlements; (iii) the effect of changes in tax laws, accounting principles, or other laws or regulatory rules affecting reported results; (iv) any reorganization and restructuring programs; (v) unusual and/or infrequently occurring items as described in Accounting Principles Board Opinion No. 30 (or any successor pronouncement thereto) and/or in management’s discussion and analysis of financial condition and results of operations appearing in the Company’s annual report to shareholders for the applicable year; (vi) acquisitions or divestitures; (vii) discontinued operations; (viii) any other specific unusual or infrequently occurring or non-recurring events, or objectively determinable category thereof; (ix) foreign exchange gains and losses; and (x) a change in the Company’s fiscal year.
(e) Terms and Conditions to Receipt of Payment. Unless otherwise provided in the applicable Award agreement, a Participant must be employed by the Company on the last day of a Performance Period to be eligible for payment in respect of a Performance Compensation Award for such Performance Period. A Participant shall be eligible to receive payment in respect of a Performance Compensation Award only to the extent that: (A) the Performance Goals for such period are achieved; and (B) all or some of the portion of such Participant’s Performance Compensation Award has been earned for the Performance Period based on the application of the Performance Formula to such achieved Performance Goals. Following the completion of a Performance Period, the Committee shall determine whether, and to what extent, the Performance Goals for the Performance Period have been achieved and, if so, calculate the amount of the Performance Compensation Awards earned for the period based upon the Performance Formula. The Committee shall then determine the amount of each Participant’s Performance Compensation Award actually payable for the Performance Period.
Annex I-11
(f) Timing of Award Payments. Except as provided in an Award agreement, Performance Compensation Awards granted for a Performance Period shall be paid to Participants as soon as administratively practicable following the Committee’s determination in accordance with Section 11(e).
12. Changes in Capital Structure and Similar Events. In the event of (i) any dividend (other than ordinary cash dividends) or other distribution (whether in the form of cash, Common Shares, other securities or other property), recapitalization, stock split, reverse stock split, reorganization, merger, amalgamation, consolidation, spin-off, split-up, split-off, combination, repurchase or exchange of Common Shares or other securities of the Company, issuance of warrants or other rights to acquire Common Shares or other securities of the Company, or other similar corporate transaction or event (including, without limitation, a Change in Control) that affects the Common Shares, or (ii) unusual or infrequently occurring events (including, without limitation, a Change in Control) affecting the Company, any Affiliate, or the financial statements of the Company or any Affiliate, or changes in applicable rules, rulings, regulations or other requirements of any governmental body or securities exchange or inter-dealer quotation system, accounting principles or law, such that in either case an adjustment is determined by the Committee in its sole discretion to be necessary or appropriate, then the Committee shall make any such adjustments in such manner as it may deem equitable, including without limitation any or all of the following:
(a) adjusting any or all of (A) the number of Common Shares or other securities of the Company (or number and kind of other securities or other property) that may be delivered in respect of Awards or with respect to which Awards may be granted under the Plan (including, without limitation, adjusting any or all of the limitations under Section 5 of the Plan) and (B) the terms of any outstanding Award, including, without limitation, (1) the number of Common Shares or other securities of the Company (or number and kind of other securities or other property) subject to outstanding Awards or to which outstanding Awards relate, (2) the Exercise Price or Strike Price with respect to any Award or (3) any applicable performance measures (including, without limitation, Performance Criteria and Performance Goals);
(b) providing for a substitution or assumption of Awards in a manner that substantially preserves the applicable terms of such Awards;
(c) accelerating the exercisability or vesting of, lapse of restrictions on, or termination of, Awards or providing for a period of time for exercise prior to the occurrence of such event;
(d) modifying the terms of Awards to add events, conditions or circumstances (including termination of employment within a specified period after a Change in Control) upon which the exercisability or vesting of or lapse of restrictions thereon will accelerate;
(e) deeming any performance measures (including, without limitation, Performance Criteria and Performance Goals) satisfied at target, maximum or actual performance through closing or such other level determined by the Committee in its sole discretion, or providing for the performance measures to continue (as is or as adjusted by the Committee) after closing;
(f) providing that for a period prior to the Change in Control determined by the Committee in its sole discretion, any Options or SARs that would not otherwise become exercisable prior to the Change in Control will be exercisable as to all Common Shares subject thereto (but any such exercise will be contingent upon and subject to the occurrence of the Change in Control and if the Change in Control does not take place after giving such notice for any reason whatsoever, the exercise will be null and void) and that any Options or SARs not exercised prior to the consummation of the Change in Control will terminate and be of no further force and effect as of the consummation of the Change in Control; and
(g) canceling any one or more outstanding Awards and causing to be paid to the holders thereof, in cash, Common Shares, other securities or other property, or any combination thereof, the value of such Awards, if any, as determined by the Committee (which if applicable may be based upon the price per Common Share received or to be received by other shareholders of the Company in such event), including without limitation, in the case of an outstanding Option or SAR, a cash payment in an amount equal to the excess, if any, of the fair market value (as of a date specified by the Committee) of the Common Shares subject to such Option or SAR over the aggregate Exercise Price or Strike Price of such Option or SAR, respectively (it being understood that, in such event, any Option or SAR having a per share Exercise Price or Strike Price equal to, or in excess of, the fair market value of a Common Share subject thereto may be canceled and terminated without any payment or consideration therefor); provided, however,
Annex I-12
that in the case of any “equity restructuring” (within the meaning of the Financial Accounting Standards Board Accounting Standards Codification Topic 718), the Committee shall make an equitable or proportionate adjustment to outstanding Awards to reflect such equity restructuring. The Company shall give each Participant notice of an adjustment hereunder and, upon notice, such adjustment shall be conclusive and binding for all purposes.
13. Amendments and Termination.
(a) Amendment and Termination of the Plan. The Board may amend, alter, suspend, discontinue, or terminate the Plan or any portion thereof at any time; provided that (i) no amendment to Section 13(b) (to the extent required by the proviso in such Section 13(b)) shall be made without shareholder approval and (ii) no such amendment, alteration, suspension, discontinuation or termination shall be made without shareholder approval if such approval is necessary to comply with any tax or regulatory requirement applicable to the Plan (including, without limitation, as necessary to comply with any rules or requirements of any securities exchange or inter-dealer quotation system on which the Common Shares may be listed or quoted); provided, further, that any such amendment, alteration, suspension, discontinuance or termination that would materially and adversely affect the rights of any Participant or any holder or beneficiary of any Award theretofore granted shall not to that extent be effective without the consent of the affected Participant, holder or beneficiary.
(b) Amendment of Award Agreements. The Committee may, to the extent consistent with the terms of any applicable Award agreement, waive any conditions or rights under, amend any terms of, or alter, suspend, discontinue, cancel or terminate, any Award theretofore granted or the associated Award agreement, prospectively or retroactively; provided that any such waiver, amendment, alteration, suspension, discontinuance, cancellation or termination that would materially and adversely affect the rights of any Participant with respect to any Award theretofore granted shall not to that extent be effective without the consent of the affected Participant; provided, further, that without shareholder approval, except as otherwise permitted under Section 12 of the Plan, (i) no amendment or modification may reduce the Exercise Price of any Option or the Strike Price of any SAR, (ii) the Committee may not cancel any outstanding Option or SAR where the Fair Market Value of the Common Shares underlying such Option or SAR is less than its Exercise Price and replace it with a new Option or SAR, another Award or cash and (iii) the Committee may not take any other action that is considered a “repricing” for purposes of the shareholder approval rules of the applicable securities exchange or inter-dealer quotation system on which the Common Shares are listed or quoted.
14. General.
(a) Award Agreements. Each Award under the Plan shall be evidenced by an Award agreement, which shall be delivered to the Participant (whether in paper or electronic medium (including email or the posting on a web site maintained by the Company or a third party under contract with the Company)) and shall specify the terms and conditions of the Award and any rules applicable thereto, including without limitation, the effect on such Award of the death, disability or termination of employment or service of a Participant, or of such other events as may be determined by the Committee.
(b) Non-Transferability.
(i) Each Award shall be exercisable only by a Participant during the Participant’s lifetime, or, if permissible under applicable law, by the Participant’s legal guardian or representative. No Award may be assigned, alienated, pledged, attached, sold or otherwise transferred or encumbered by a Participant other than by will or by the laws of descent and distribution and any such purported assignment, alienation, pledge, attachment, sale, transfer or encumbrance shall be void and unenforceable against the Company or an Affiliate; provided that the designation of a beneficiary shall not constitute an assignment, alienation, pledge, attachment, sale, transfer or encumbrance.
(ii) Notwithstanding the foregoing, the Committee may, in its sole discretion, permit Awards (other than Incentive Stock Options) to be transferred by a Participant, without consideration, subject to such rules as the Committee may adopt consistent with any applicable Award agreement to preserve the purposes of the Plan, to: (A) any person who is a “family member” of the Participant, as such term is used in the instructions to Form S-8 under the Securities Act (collectively, the “Immediate Family Members”); (B) a trust solely for the benefit of the Participant and his or her Immediate Family Members; (C) a partnership or limited liability company whose only partners or stockholders are the Participant and his or her Immediate Family Members; or (D) any other transferee as may be approved either (I) by the Board or the Committee in its sole discretion, or (II) as provided in the applicable
Annex I-13
Award agreement. (each transferee described in clauses (A), (B), (C) and (D) above is hereinafter referred to as a “Permitted Transferee”); provided that the Participant gives the Committee advance written notice describing the terms and conditions of the proposed transfer and the Committee notifies the Participant in writing that such a transfer would comply with the requirements of the Plan.
(iii) The terms of any Award transferred in accordance with the immediately preceding sentence shall apply to the Permitted Transferee and any reference in the Plan, or in any applicable Award agreement, to a Participant shall be deemed to refer to the Permitted Transferee, except that (A) Permitted Transferees shall not be entitled to transfer any Award, other than by will or the laws of descent and distribution; (B) Permitted Transferees shall not be entitled to exercise any transferred Option unless there shall be in effect a registration statement on an appropriate form covering the Common Shares to be acquired pursuant to the exercise of such Option if the Committee determines, consistent with any applicable Award agreement, that such a registration statement is necessary or appropriate; (C) the Committee or the Company shall not be required to provide any notice to a Permitted Transferee, whether or not such notice is or would otherwise have been required to be given to the Participant under the Plan or otherwise; and (D) the consequences of the termination of the Participant’s employment by, or services to, the Company or an Affiliate under the terms of the Plan and the applicable Award agreement shall continue to be applied with respect to the Participant, including, without limitation, that an Option shall be exercisable by the Permitted Transferee only to the extent, and for the periods, specified in the Plan and the applicable Award agreement.
(c) Tax Withholding and Deductions.
(i) A Participant shall be required to pay to the Company or any Affiliate, and the Company or any Affiliate shall have the right and is hereby authorized to deduct and withhold, from any cash, Common Shares, other securities or other property deliverable under any Award or from any compensation or other amounts owing to a Participant, the amount (in cash, Common Shares, other securities or other property) of any required taxes (up to the maximum statutory rate under applicable law as in effect from time to time as determined by the Committee) and deduction in respect of an Award, its grant, vesting or exercise, or any payment or transfer under an Award or under the Plan and to take such other action as may be necessary in the opinion of the Committee or the Company to satisfy all obligations for the payment of such taxes.
(ii) Without limiting the generality of clause (i) above, the Committee may, in its sole discretion, determined on a case by case basis, permit a Participant to satisfy, in whole or in part, the foregoing tax and deduction liability by (A) the delivery of Common Shares (which are not subject to any pledge or other security interest and are Mature Shares, except as otherwise determined by the Committee) owned by the Participant having a fair market value equal to such liability or (B) having the Company withhold from the number of Common Shares otherwise issuable or deliverable pursuant to the exercise or settlement of the Award a number of shares with a Fair Market Value equal to such liability.
(d) No Claim to Awards; No Rights to Continued Employment; Waiver. No employee of the Company or an Affiliate, or other person, shall have any claim or right to be granted an Award under the Plan or, having been selected for the grant of an Award, to be selected for a grant of any other Award. There is no obligation for uniformity of treatment of Participants or holders or beneficiaries of Awards. The terms and conditions of Awards and the Committee’s determinations and interpretations with respect thereto need not be the same with respect to each Participant and may be made selectively among Participants, whether or not such Participants are similarly situated. Neither the Plan nor any action taken hereunder shall be construed as giving any Participant any right to be retained in the employ or service of the Company or an Affiliate, nor shall it be construed as giving any Participant any rights to continued service on the Board. The Company or any of its Affiliates may at any time dismiss a Participant from employment or discontinue any consulting relationship, free from any liability or any claim under the Plan, unless otherwise expressly provided in the Plan or any Award agreement. By accepting an Award under the Plan, a Participant shall thereby be deemed to have waived any claim to continued exercise or vesting of an Award or to damages or severance entitlement related to non-continuation of the Award beyond the period provided under the Plan or any Award agreement, notwithstanding any provision to the contrary in any written employment contract or other agreement between the Company and its Affiliates and the Participant, whether any such agreement is executed before, on or after the Date of Grant.
(e) Addenda. The Committee may adopt such addenda to the Plan as it may consider necessary or appropriate for the purpose of granting Awards, which Awards may contain such terms and conditions as the Committee deems necessary or appropriate to accommodate differences in local law, tax policy or custom, which may deviate from the terms and conditions set forth in this Plan. The terms of any such addenda shall supersede the terms
Annex I-14
of the Plan to the extent necessary to accommodate such differences but shall not otherwise affect the terms of the Plan as in effect for any other purpose. With respect to Participants who reside or work outside of the United States of America, the Committee may in its sole discretion amend the terms of the Plan or outstanding Awards with respect to such Participants in order to conform such terms with the requirements of local law or to obtain more favorable tax or other treatment for a Participant, the Company or its Affiliates.
(f) Designation and Change of Beneficiary. Each Participant may file with the Committee a written designation of one or more persons as the beneficiary(ies) who shall be entitled to receive the amounts payable with respect to an Award, if any, due under the Plan upon his death. A Participant may, from time to time, revoke or change his beneficiary designation without the consent of any prior beneficiary by filing a new designation with the Committee. The last such designation received by the Committee shall be controlling; provided, however, that no designation, or change or revocation thereof, shall be effective unless received by the Committee prior to the Participant’s death, and in no event shall it be effective as of a date prior to such receipt. If no beneficiary designation is filed by a Participant, the beneficiary shall be deemed to be his or her spouse or, if the Participant is unmarried at the time of death, his or her estate.
(g) Termination of Employment/Service. Unless determined otherwise by the Committee at any point following such event: (i) neither a temporary absence from employment or service due to illness, vacation or leave of absence nor a transfer from employment or service with the Company to employment or service with an Affiliate (or vice-versa) shall be considered a termination of employment or service with the Company or an Affiliate; and (ii) if a Participant’s employment with the Company and its Affiliates terminates, but such Participant continues to provide services to the Company and its Affiliates in a non-employee capacity (or vice-versa), such change in status shall not be considered a termination of employment with the Company or an Affiliate.
(h) No Rights as a Stockholder. Except as otherwise specifically provided in the Plan or any Award agreement, no person shall be entitled to the privileges of ownership in respect of Common Shares or other securities that are subject to Awards hereunder until such shares have been issued or delivered to that person.
(i) Government and Other Regulations.
(i) The obligation of the Company to settle Awards in Common Shares or other consideration shall be subject to all applicable laws, rules, and regulations, and to such approvals by governmental agencies as may be required. Notwithstanding any terms or conditions of any Award to the contrary, the Company shall be under no obligation to offer to sell or to sell, and shall be prohibited from offering to sell or selling, any Common Shares or other securities pursuant to an Award unless such shares have been properly registered for sale pursuant to the Securities Act with the Securities and Exchange Commission or unless the Company has received an opinion of counsel, satisfactory to the Company, that such shares may be offered or sold without such registration pursuant to an available exemption therefrom and the terms and conditions of such exemption have been fully complied with. The Company shall be under no obligation to register for sale under the Securities Act any of the Common Shares or other securities to be offered or sold under the Plan. The Committee shall have the authority to provide that all certificates for Common Shares or other securities of the Company or any Affiliate delivered under the Plan shall be subject to such stop transfer orders and other restrictions as the Committee may deem advisable under the Plan, the applicable Award agreement, the federal securities laws, or the rules, regulations and other requirements of the Securities and Exchange Commission, any securities exchange or inter-dealer quotation system upon which such shares or other securities are then listed or quoted and any other applicable federal, state, local or non-U.S. laws, and, without limiting the generality of Section 9 of the Plan, the Committee may cause a legend or legends to be put on any such certificates to make appropriate reference to such restrictions. Notwithstanding any provision in the Plan to the contrary, the Committee reserves the right to add any additional terms or provisions to any Award granted under the Plan that it in its sole discretion deems necessary or advisable in order that such Award complies with the legal requirements of any governmental entity to whose jurisdiction the Award is subject.
(ii) The Committee may cancel an Award or any portion thereof if it determines, in its sole discretion, that legal or contractual restrictions and/or blockage and/or other market considerations would make the Company’s acquisition of Common Shares from the public markets, the Company’s issuance of Common Shares or other securities to the Participant, the Participant’s acquisition of Common Shares or other securities from the Company and/or the Participant’s sale of Common Shares to the public markets, illegal, impracticable or inadvisable. If the Committee determines to cancel all or any portion of an Award denominated in Common Shares in accordance with the foregoing, the Company shall pay to the Participant an amount equal to the excess of (A) the aggregate fair market value of the Common Shares subject to such Award or portion thereof canceled (determined as of the applicable exercise date, or the date that the shares would have been vested or delivered, as applicable), over (B) the aggregate
Annex I-15
Exercise Price or Strike Price (in the case of an Option or SAR, respectively) or any amount payable as a condition of delivery of Common Shares (in the case of any other Award). Such amount shall be delivered to the Participant as soon as practicable following the cancellation of such Award or portion thereof.
(j) Payments to Persons Other Than Participants. If the Committee shall find that any person to whom any amount is payable under the Plan is unable to care for his affairs because of illness or accident, or is a minor, or has died, then any payment due to such person or his estate (unless a prior claim therefor has been made by a duly appointed legal representative) may, if the Committee so directs the Company, be paid to his spouse, child, relative, an institution maintaining or having custody of such person, or any other person deemed by the Committee to be a proper recipient on behalf of such person otherwise entitled to payment. Any such payment shall be a complete discharge of the liability of the Committee and the Company therefor.
(k) Non-Exclusivity of the Plan. Neither the adoption of this Plan by the Board nor the submission of this Plan to the shareholders of the Company for approval shall be construed as creating any limitations on the power of the Board to adopt such other incentive arrangements as it may deem desirable, including, without limitation, the granting of stock options or other equity-based awards otherwise than under this Plan, and such arrangements may be either applicable generally or only in specific cases.
(l) No Trust or Fund Created. Neither the Plan nor any Award shall create or be construed to create a trust or separate fund of any kind or a fiduciary relationship between the Company or any Affiliate, on the one hand, and a Participant or other person or entity, on the other hand. No provision of the Plan or any Award shall require the Company, for the purpose of satisfying any obligations under the Plan, to purchase assets or place any assets in a trust or other entity to which contributions are made or otherwise to segregate any assets, nor shall the Company maintain separate bank accounts, books, records or other evidence of the existence of a segregated or separately maintained or administered fund for such purposes. Participants shall have no rights under the Plan other than as unsecured general creditors of the Company, except that insofar as they may have become entitled to payment of additional compensation by performance of services, they shall have the same rights as other employees under general law.
(m) Reliance on Reports. Each member of the Committee and each member of the Board shall be fully justified in acting or failing to act, as the case may be, and shall not be liable for having so acted or failed to act in good faith, in reliance upon any report made by the independent public accountant of the Company and its Affiliates and/or any other information furnished in connection with the Plan by any agent of the Company or the Committee or the Board, other than himself.
(n) Relationship to Other Benefits. No payment under the Plan shall be taken into account in determining any benefits under any pension, retirement, profit sharing, group insurance or other benefit plan of the Company except as otherwise specifically provided in such other plan.
(o) Governing Law. The Plan shall be governed by and construed in accordance with the laws of the State of Delaware, without giving effect to the conflict of laws provisions thereof. Each party hereby irrevocably and unconditionally submits, for itself and its property, to the exclusive jurisdiction of the state and federal courts seated in Delaware (and any appellate courts thereof) in any action or proceeding arising out of or relating to this Plan, and each of the parties hereby irrevocably and unconditionally (a) agrees not to commence any such action or proceeding except in such courts, (b) agrees that any claim in respect of any such action or proceeding may be heard and determined in such court, (c) waives, to the fullest extent it may legally and effectively do so, any objection which it may now or hereafter have to the laying of venue of any such action or proceeding in any such court, and (d) waives, to the fullest extent permitted by law, the defense of an inconvenient forum to the maintenance of such action or proceeding in any such court. Each party agrees that a final judgment in any such action or proceeding shall be conclusive and may be enforced in other jurisdictions by suit on the judgment or in any other manner provided by law. Each party hereby knowingly, voluntarily and intentionally irrevocably waives the right to a trial by jury in respect to any litigation, dispute, claim, legal action or other legal proceeding based hereon, or arising out of, under, or in connection with, this Plan.
(p) Severability. If any provision of the Plan or any Award or Award agreement is or becomes or is deemed to be invalid, illegal, or unenforceable in any jurisdiction or as to any person or entity or Award, or would disqualify the Plan or any Award under any law deemed applicable by the Committee, such provision shall be construed or deemed amended to conform to the applicable laws, or if it cannot be construed or deemed amended without, in the determination of the Committee, materially altering the intent of the Plan or the Award, such provision shall be construed or deemed stricken as to such jurisdiction, person or entity or Award and the remainder of the Plan and any such Award shall remain in full force and effect.
Annex I-16
(q) Obligations Binding on Successors. The obligations of the Company under the Plan shall be binding upon any successor corporation or organization resulting from the merger, amalgamation, consolidation or other reorganization of the Company, or upon any successor corporation or organization succeeding to substantially all of the assets and business of the Company.
(r) Code Section 409A.
(i) Notwithstanding any provision of this Plan to the contrary, all Awards made under this Plan are intended to be exempt from or, in the alternative, comply with Code Section 409A and the interpretive guidance thereunder, including the exceptions for stock rights and short-term deferrals. The Plan shall be construed and interpreted in accordance with such intent. Each payment under an Award shall be treated as a separate payment for purposes of Code Section 409A and, if an Award includes “dividend equivalents” within the meaning of Section 1.409A-3(e) of Code Section 409A, the Participant’s right to receive the dividend equivalents will be treated separately from the right to other amounts under the Award.
(ii) If a Participant is a “specified employee” (as such term is defined for purposes of Code Section 409A) at the time of his or her termination of service, no amount that is non-qualified deferred compensation subject to Code Section 409A and that becomes payable by reason of such termination of service shall be paid to the Participant (or in the event of the Participant’s death, the Participant’s representative or estate) before the earlier of (x) the first business day after the date that is six months following the date of the Participant’s termination of service, and (y) within thirty (30) days following the date of the Participant’s death (in each case, without interest). Any payments of non-qualified deferred compensation under any Award payable more than six months following the Participant’s termination of service will be paid at the time or times the payments are otherwise scheduled to be made. For purposes of Code Section 409A, a termination of service shall be deemed to occur only if it is a “separation from service” within the meaning of Code Section 409A, and references in the Plan and any Award agreement to “termination of service” or similar terms shall mean a “separation from service”, whether such “separation from service” occurs upon or after the Participant’s termination of service. If any Award is or becomes subject to Code Section 409A and if payment of such Award would be accelerated or otherwise triggered under a Change in Control, then the definition of Change in Control shall be deemed modified, only to the extent necessary to avoid the imposition of an excise tax under Code Section 409A, to mean a “change in control event” as such term is defined for purposes of Code Section 409A.
(iii) Any adjustments made pursuant to Section 12 to Awards that are subject to Code Section 409A shall be made in compliance with the requirements of Code Section 409A, and any adjustments made pursuant to Section 12 to Awards that are not subject to Code Section 409A shall be made in such a manner as to ensure that after such adjustment, the Awards either (x) continue not to be subject to Code Section 409A or (y) comply with the requirements of Code Section 409A.
(s) Expenses; Gender; Titles and Headings. The expenses of administering the Plan shall be borne by the Company and its Affiliates. Masculine pronouns and other words of masculine gender shall refer to both men and women. The titles and headings of the sections in the Plan are for convenience of reference only, and in the event of any conflict, the text of the Plan, rather than such titles or headings shall control.
(t) Other Agreements. Notwithstanding the above, the Committee may require, as a condition to the grant of and/or the receipt of Common Shares or other securities under an Award, that the Participant execute lock-up, shareholder or other agreements, as it may determine in its sole and absolute discretion.
(u) Payments. Participants shall be required to pay, to the extent required by applicable law, any amounts required to receive Common Shares or other securities under any Award made under the Plan.
(v) Erroneously Awarded Compensation. All Awards shall be subject (including on a retroactive basis) to (i) any clawback, forfeiture or similar incentive compensation recoupment policy established from time to time by the Company, including, without limitation, any such policy established to comply with the Dodd-Frank Wall Street Reform and Consumer Protection Act, (ii) applicable law (including, without limitation, Section 304 of the Sarbanes-Oxley Act and Section 954 of the Dodd-Frank Wall Street Reform and Consumer Protection Act), and/or (iii) the rules and regulations of the applicable securities exchange or inter-dealer quotation system on which the Common Shares or other securities are listed or quoted, and such requirements shall be deemed incorporated by reference into all outstanding Award agreements.
[Signature page follows]
Annex I-17
IN WITNESS WHEREOF, this Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan has been duly approved and adopted by the Company and the shareholders as of the dates set forth below.
Adopted by consent of the Board: September 18, 2024
Shareholder Approved: September 18, 2024
|
APIMEDS PHARMACEUTICALS US, INC. |
||||
|
By: |
/s/ Erik Emerson |
|||
|
Title: |
Chief Executive Officer |
|||
|
Date: |
September 18, 2024 |
|||
[Signature page to Apimeds Pharmaceuticals US, Inc. 2024 Equity Incentive Plan]
Annex I-18
APIMEDS PHARMACEUTICALS US, INC.
2025 EQUITY INCENTIVE PLAN
1. Purpose. The purposes of this Plan are to:
(a) attract, retain, and motivate Employees, Directors, and Consultants,
(b) provide additional incentives to Employees, Directors, and Consultants, and
(c) promote the success of the Company’s business,
by providing Employees, Directors, and Consultants with opportunities to acquire the Company’s Shares, or to receive monetary payments based on the value of such Shares. Additionally, the Plan is intended to assist in further aligning the interests of the Company’s Employees, Directors, and Consultants to those of its shareholders.
2. Definitions. As used herein, the following definitions will apply:
(a) “Administrator” means a committee of at least one Director of the Company as the Board may appoint to administer this Plan or, if no such committee has been appointed by the Board, the Board.
(b) “Applicable Laws” means the requirements relating to the administration of equity-based awards or equity compensation plans under U.S. state corporate laws, U.S. federal and state securities laws, the Code, any stock exchange or quotation system on which the Shares are listed or quoted and the applicable laws of any foreign country or jurisdiction where Awards are, or will be, granted under the Plan.
(c) “Award” means, individually or collectively, a grant under the Plan of Stock Options, Stock Appreciation Rights, Restricted Stock, Restricted Stock Units, or Other Stock-Based Awards.
(d) “Award Agreement” means the written or electronic agreement, consistent with the terms of the Plan, between the Company and the Participant, setting forth the terms, conditions, and restrictions applicable to each Award.
(e) “Board” means the Company’s Board of Directors, as constituted from time to time and, where the context so requires, reference to the “Board” may refer to a committee to whom the Board has delegated authority to administer any aspect of this Plan.
(f) “Cause” shall have the meaning ascribed to such term, or term of similar effect, in any offer letter, employment, consulting, severance, or similar agreement, including any Award Agreement, between the Participant and the Company or any Subsidiary; provided, that in the absence of an offer letter, employment, consulting, severance, or similar agreement containing such definition, “Cause” means:
(i) any willful, material violation by the Participant of any law or regulation applicable to the business of the Company, a Subsidiary, or other affiliate of the Company;
(ii) the Participant’s conviction for, or guilty plea to, a felony (or crime of similar magnitude under Applicable Laws outside the United States) or a crime involving moral turpitude, or any willful perpetration by the Participant of a common law fraud, act of material dishonesty, embezzlement, or misappropriation or similar conduct against the Company, a Subsidiary, or other affiliate of the Company;
(iii) the Participant’s commission of an act of personal dishonesty which involves personal profit in connection with the Company, a Subsidiary, other affiliate of the Company, or any other entity having a business relationship with any of the foregoing;
(iv) any material breach or violation by the Participant of any fiduciary duties or duties of care to the Company or provision of any agreement or understanding between the Company, a Subsidiary, or other affiliate of the Company and the Participant regarding the terms of the Participant’s Service, including without limitation, the willful and continued failure or refusal of the Participant to perform
Annex J-1
the material duties required of such Participant as an Employee, Director, or Consultant of the Company, a Subsidiary, or other affiliate of the Company, other than as a result of having a Disability, or a breach of any applicable invention assignment, confidentiality, non-competition, non-solicitation, restrictive covenant, or similar agreement between the Company, a Subsidiary, or other affiliate of the Company and the Participant;
(v) any willful and continued refusal by the Participant to carry out a reasonable directive of the chief executive officer, the Board or the Participant’s direct supervisor, which involves the business of the Company, a Subsidiary, or other affiliate of the Company and was capable of being lawfully performed;
(vi) the Participant’s violation of the code of ethics of the Company or any Subsidiary;
(vii) the Participant’s disregard of the policies of the Company, a Subsidiary, or other affiliate of the Company so as to cause loss, harm, damage, or injury to the property, reputation, or employees of the Company, a Subsidiary, or other affiliate of the Company; or
(viii) any other misconduct by the Participant that is injurious to the financial condition or business reputation of, or is otherwise injurious to, the Company, a Subsidiary, or other affiliate of the Company;
provided that, in the case of clauses (iv), (v), (vi), (vii), and (viii) above, the Company has given written notice to the Participant of such circumstances and which circumstances, if capable of being cured, has not been cured within thirty (30) days after such notice.
(g) “Change in Control” means the occurrence of any of the following events:
(i) any “person” (as such term is used in Sections 13(d) and 14(d) of the Exchange Act) becomes the “beneficial owner” (as defined in Rule 13d-3 of the Exchange Act), directly or indirectly, of securities of the Company representing fifty percent (50%) or more of the total voting power represented by the Company’s then outstanding voting securities;
(ii) the consummation of the sale or disposition by the Company of all or substantially all of the Company’s assets;
(iii) a change in the composition of the Board occurring within a two-year period, as a result of which fewer than a majority of the Directors are Incumbent Directors. “Incumbent Directors” means directors who either (A) are Directors as of the Effective Date, or (B) are elected, or nominated for election, to the Board with the affirmative votes of at least a majority of the Incumbent Directors at the time of such election or nomination (but will not include an individual whose election or nomination is in connection with an actual or threatened proxy contest relating to the election of Directors); or
(iv) the consummation of a merger or consolidation of the Company with any other corporation, other than a merger or consolidation which would result in the voting securities of the Company outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity or its parent) at least fifty percent (50%) of the total voting power represented by the voting securities of the Company or such surviving entity or its parent outstanding immediately after such merger or consolidation.
Notwithstanding the foregoing, a transaction shall not constitute a Change in Control if its sole purpose is to change the jurisdiction of the Company’s incorporation or to create a holding company that will be owned in substantially the same proportions by the persons who held the Company’s securities immediately before such transaction. In addition, if a Change in Control constitutes a payment event with respect to any Award which provides for a deferral of compensation and is subject to Code Section 409A, then notwithstanding anything to the contrary in the Plan or applicable Award Agreement, the transaction with respect to such Award must also constitute a “change in control event” as defined in Treasury Regulation Section 1.409A-3(i)(5) to the extent required by Code Section 409A.
(h) “Code” means the Internal Revenue Code of 1986, as amended. Any reference to a section of the Code herein will be a reference to any successor or amended section of the Code.
(i) “Company” means Apimeds Pharmaceuticals US, Inc., a Delaware corporation, or any successor thereto.
Annex J-2
(j) “Consultant” means a consultant or adviser who provides bona fide services to the Company, its Parent, or any Subsidiary as an independent contractor and who qualifies as a consultant or advisor under Instruction A.1.(a)(1) of Form S-8 under the Securities Act.
(k) “Director” means a member of the Board.
(l) “Disability” means total and permanent disability as defined in Code Section 22(e)(3), provided that in the case of an Award other than an Incentive Stock Option, the Administrator in its discretion may determine whether a permanent and total disability exists in accordance with uniform and non-discriminatory standards adopted by the Administrator from time to time.
(m) “Effective Date” shall have the meaning set forth in Section 24.
(n) “Employee” means any person, including officers and Directors, employed by the Company, its Parent, or any Subsidiary. Neither Service as a Director nor payment of a director’s fee by the Company will be sufficient to constitute “employment” by the Company.
(o) “Exchange Act” means the Securities Exchange Act of 1934, as amended.
(p) “Fair Market Value” means, as of any date, the value of a Share, determined as follows:
(i) if the Shares are readily tradable on an established securities market, its Fair Market Value will be the closing sales price for such shares (or the closing bid, if no sales were reported) as quoted on such market for the day of determination, as reported in The Wall Street Journal or such other source as the Administrator deems reliable;
(ii) if the Shares are regularly quoted by a recognized securities dealer but selling prices are not reported, its Fair Market Value will be the mean between the high bid and low asked prices for a Share for the day of determination, as reported in The Wall Street Journal or such other source as the Administrator deems reliable; or
(iii) if the Shares are not readily tradable on an established securities market, the Fair Market Value will be determined in good faith by the Administrator.
Notwithstanding the preceding, for federal, state, and local income tax reporting purposes and for such other purposes as the Administrator deems appropriate, Fair Market Value shall be determined by the Administrator in accordance with uniform and nondiscriminatory standards adopted by it from time to time. In addition, the determination of Fair Market Value in all cases shall be in accordance with the requirements set forth under Code Section 409A to the extent necessary for an Award to comply with, or be exempt from, Code Section 409A. The Administrator’s determination shall be conclusive and binding on all persons.
(q) “Incentive Stock Option” means a Stock Option intended to qualify as an incentive stock option within the meaning of Code Section 422 and the regulations promulgated thereunder.
(r) “Non-Employee Director” means a Director who is a “non-employee director” within the meaning of Exchange Act Rule 16b-3.
(s) “Nonqualified Stock Option” means a Stock Option that by its terms, or in operation, does not qualify or is not intended to qualify as an Incentive Stock Option.
(t) “Other Stock-Based Awards” means any other awards not specifically described in the Plan that are valued in whole or in part by reference to, or are otherwise based on, Shares and are created by the Administrator pursuant to Section 11.
(u) “Parent” means a “parent corporation,” whether now or hereafter existing, as defined in Code Section 424(e).
(v) “Participant” means the holder of an outstanding Award.
(w) “Period of Restriction” means the period during which the transfer of Restricted Stock is subject to restrictions and a substantial risk of forfeiture. Such restrictions may be based on the passage of time, the achievement of certain performance criteria, or the occurrence of other events as determined by the Administrator.
Annex J-3
(x) “Plan” means this Apimeds Pharmaceuticals US, Inc. 2025 Equity Incentive Plan.
(y) “Restricted Stock” means Shares, subject to a Period of Restriction or certain other specified restrictions (including, without limitation, a requirement that the Participant remain continuously employed or provide continuous Service for a specified period of time), granted under Section 9 or issued pursuant to the early exercise of a Stock Option.
(z) “Restricted Stock Unit” or “RSU” means an unfunded and unsecured promise to deliver Shares, cash, other securities, or other property, subject to certain restrictions (including, without limitation, a requirement that the Participant remain continuously employed or provide continuous Service for a specified period of time), granted under Section 10.
(aa) “Service” means service as a Service Provider. In the event of any dispute over whether and when Service has terminated, the Administrator shall have sole discretion to determine whether such termination has occurred and the effective date of such termination.
(bb) “Service Provider” means an Employee, Director, or Consultant, including any prospective Employee, Director, or Consultant who has accepted an offer of employment or Service and will be an Employee, Director, or Consultant after the commencement of their Service.
(cc) “Shares” means the Company’s shares of common stock, par value of $0.01 per share.
(dd) “Stock Appreciation Right” or “SAR” means an Award pursuant to Section 8 that is designated as a SAR.
(ee) “Stock Option” means an option granted pursuant to the Plan to purchase Shares, whether designated as an Incentive Stock Option or a Nonqualified Stock Option.
(ff) “Subsidiary” means a “subsidiary corporation,” whether now or hereafter existing, as defined in Code Section 424(f).
(gg) “Substitute Award” has the meaning set forth in Section 4(i).
3. Awards.
(a) Award Types. The Plan permits the grant of Stock Options, Stock Appreciation Rights, Restricted Stock, Restricted Stock Units, and Other Stock-Based Awards.
(b) Award Agreements. Awards shall be evidenced by Award Agreements (which need not be identical) in such forms as the Administrator may from time to time approve; provided, however, that in the event of any conflict between the provisions of the Plan and any such Award Agreements, the provisions of the Plan shall prevail.
(c) Date of Grant. The date of grant of an Award will be, for all purposes, the date on which the Administrator makes the determination granting such Award, or such later date as is determined by the Administrator, consistent with Applicable Laws. Notice of the determination will be provided to each Participant within a reasonable time after the date of such grant.
4. Shares Available for Awards.
(a) Basic Limitation. Subject to the provisions of Section 14, the maximum aggregate number of Shares that may be issued under the Plan is ten percent (10%) of the Shares outstanding on [ ], 2025 (the “Plan Share Limit”). The Shares subject to the Plan may be authorized, but unissued, or reacquired shares.
(b) Annual Increase in Available Shares. On the first day of each calendar year during the term of the Plan, commencing on January 1, 2026 and continuing until (and including) January 1, 2035, the number of Shares available under the Plan Share Limit shall automatically increase by a number equal to the lesser of (i) three percent (3%) of the total number of Shares issued and outstanding on December 31 of the calendar year immediately preceding the date of such increase and (ii) a number of Shares determined by the Board.
(c) Awards Not Settled in Shares Delivered to Participant. Upon payment in Shares pursuant to the exercise or settlement of an Award, the number of Shares available for issuance under the Plan shall be reduced only by the number of Shares actually issued in such payment. If a Participant pays the exercise price
Annex J-4
(or purchase price, if applicable) of an Award through the tender of Shares, or if the Shares are tendered or withheld to satisfy any tax withholding obligations, the number of Shares so tendered or withheld shall again be available for issuance pursuant to future Awards under the Plan, although such Shares shall not again become available for issuance as Incentive Stock Options.
(d) Cash-Settled Awards. Shares shall not be deemed to have been issued pursuant to the Plan with respect to any portion of an Award that is settled in cash.
(e) Lapsed Awards. If any outstanding Award expires or is terminated or canceled without having been exercised or settled in full, or if the Shares acquired pursuant to an Award subject to forfeiture or repurchase are forfeited or repurchased by the Company, the Shares allocable to the terminated portion of such Award or such forfeited or repurchased Shares shall again be available for grant under the Plan.
(f) Code Section 422 Limitations. No more than ten percent (10%) of the Shares outstanding on [ ], 2025 (subject to adjustment pursuant to Section 14) may be issued under the Plan upon the exercise of Incentive Stock Options.
(g) Non-Employee Director Award Limit. Notwithstanding any provision to the contrary in the Plan or in any policy of the Company regarding Non-Employee Director compensation, the sum of the grant date fair value (determined as of the grant date in accordance with Financial Accounting Standards Board Accounting Standards Codification Topic 718, or any successor thereto) of all equity-based Awards and the maximum amount that may become payable pursuant to all cash-based Awards that may be granted to a Service Provider as compensation for services as a Non-Employee Director during any calendar year shall not exceed $750,000 for such Service Provider’s first year of service as a Non-Employee Director and $500,000 for each year thereafter.
(h) Share Reserve. The Company, during the term of the Plan, shall at all times keep available such number of Shares authorized for issuance as will be sufficient to satisfy the requirements of the Plan.
(i) Substitute Awards. Awards may, in the sole discretion of the Administrator, be granted under the Plan in assumption of, or in substitution for, outstanding awards previously granted by an entity acquired by the Company, its Parent, or any Subsidiary or with which the Company, its Parent, or any Subsidiary combines (“Substitute Awards”). Substitute Awards shall not be counted against the Plan Share Limit; provided, however, that Substitute Awards issued in connection with the assumption of, or in substitution for, outstanding stock options intended to qualify as Incentive Stock Options shall be counted against the Incentive Stock Option limit in Section 4(f).
5. Administration. The Plan will be administered by the Administrator.
(a) Powers of the Administrator. Subject to the provisions of the Plan, the Administrator will have the authority, in its discretion to:
(i) determine Fair Market Value;
(ii) select the Service Providers to whom Awards may be granted;
(iii) determine the type or types of Awards to be granted to Participants under the Plan and number of the Shares to be covered by each Award;
(iv) approve forms of Award Agreements for use under the Plan;
(v) determine the terms and conditions, not inconsistent with the terms of the Plan, of any Award. Such terms and conditions include, but are not limited to, the exercise price or purchase price, the time or times when Awards may be exercised (which may be based on performance criteria), any vesting criteria or Periods of Restriction, any vesting acceleration or waiver of forfeiture or repurchase restrictions, and any restriction or limitation regarding any Award or the Shares relating thereto, based in each case on such factors as the Administrator, in its sole discretion, will determine;
(vi) construe and interpret the terms of the Plan, any Award Agreement, and Awards granted pursuant to the Plan;
Annex J-5
(vii) prescribe, amend, and rescind rules and regulations relating to the Plan, including rules and regulations relating to sub-plans established for the purpose of satisfying applicable foreign laws and/or qualifying for preferred tax treatment under applicable tax laws, as well as any privacy or other notice provisions to comply with Applicable Laws;
(viii) modify or amend each Award (subject to Section 18(c)), including (A) the discretionary authority to extend the post-termination exercisability period of Awards and (B) accelerate the satisfaction of any vesting criteria or waiver of forfeiture or repurchase restrictions;
(ix) allow Participants to satisfy withholding tax obligations by electing to have the Company withhold from the Shares or cash to be issued upon exercise or vesting of an Award that number of Shares or cash having a Fair Market Value equal to the amount required to be withheld. The Fair Market Value of any Shares to be withheld will be determined on the date that the amount of tax to be withheld is to be determined. All elections by a Participant to have Shares or cash withheld for this purpose will be made in such form and under such conditions as the Administrator may deem necessary or advisable;
(x) authorize any person to execute on behalf of the Company any instrument required to effect the grant of an Award previously granted by the Administrator;
(xi) allow a Participant to defer the receipt of the payment of cash or the delivery of the Shares that would otherwise be due to such Participant under an Award, subject to compliance (or exemption) from Code Section 409A;
(xii) determine whether Awards will be settled in cash, Shares, other securities, other property, or in any combination thereof;
(xiii) determine whether Awards will be adjusted for dividend equivalents;
(xiv) create Other Stock-Based Awards for issuance under the Plan;
(xv) impose such restrictions, conditions, or limitations as it determines appropriate as to the timing and manner of any resales by a Participant or other subsequent transfers by the Participant of any securities issued as a result of or under an Award, including without limitation, (A) restrictions under an insider trading policy, and (B) restrictions as to the use of a specified brokerage firm for such resales or other transfers; and
(xvi) make all other determinations and take all other action deemed necessary or advisable for administering the Plan and due compliance with Applicable Laws, stock market or exchange rules or regulations or accounting or tax rules or regulations.
(b) Repricing Permitted. The Administrator may modify the purchase price or the exercise price of any outstanding Award, or cancel any Award in exchange for cash or another Award without the approval of the Company’s shareholders.
(c) Section 16. To the extent desirable to qualify transactions hereunder as exempt under Exchange Act Rule 16b-3, the transactions contemplated hereunder will be approved by the entire Board or a committee of two or more Non-Employee Directors.
(d) Delegation of Authority. Except to the extent prohibited by Applicable Laws, the Administrator may delegate to one or more officers of the Company some or all of its authority under the Plan, including the authority to grant all types of Awards, in accordance with Applicable Laws (except that such delegation shall not apply to any Award for a Participant then covered by Section 16 of the Exchange Act), and the Administrator may delegate to one or more committees of the Board (which may consist solely of one Director) some or all of its authority under this Plan, including the authority to grant all types of Awards, in accordance with Applicable Laws. Such delegation may be revoked at any time. The acts of such delegates shall be treated as acts of the Administrator, and such delegates shall report regularly to the Administrator regarding the delegated duties and responsibilities and any Awards granted.
Annex J-6
(e) Effect of Administrator’s Decision. The Administrator’s decisions, determinations, and interpretations will be final and binding on all persons, including Participants and any other holders of Awards.
6. Eligibility. The Administrator has the discretion to select any Service Provider to receive an Award, although Incentive Stock Options may be granted only to Employees. Designation of a Participant in any year shall not require the Administrator to designate such person to receive an Award in any other year or, once designated, to receive the same type or amount of Award as granted to the Participant in any other year. The Administrator shall consider such factors as it deems pertinent in selecting Participants and in determining the type and amount of their respective Awards.
7. Stock Options. The Administrator, at any time and from time to time, may grant Stock Options under the Plan to Service Providers. Each Stock Option shall be subject to such terms and conditions consistent with the Plan as the Administrator may impose from time to time, subject to the following limitations:
(a) Exercise Price. The per share exercise price for Shares to be issued pursuant to exercise of a Stock Option will be determined by the Administrator, but shall be no less than 100% of the Fair Market Value per Share on the date of grant, subject to Section 7(e). Notwithstanding the foregoing, in the case of a Stock Option that is a Substitute Award, the exercise price for Shares subject to such Stock Option may be less than the Fair Market Value per Share on the date of grant; provided that the exercise price of any Substitute Award shall be determined in accordance with the applicable requirements of Code Sections 424 and 409A.
(b) Exercise Period. Stock Options shall be exercisable at such time or times and subject to such terms and conditions as shall be determined by the Administrator; provided, however, that no Stock Option shall be exercisable later than ten (10) years after the date it is granted. Stock Options shall terminate at such earlier times and upon such conditions or circumstances as the Administrator shall in its discretion set forth in such Award Agreement at the date of grant; provided, however, the Administrator may, in its sole discretion, later waive any such condition.
(c) Payment of Exercise Price. To the extent permitted by Applicable Laws, the Participant may pay the Stock Option exercise price by:
(i) cash;
(ii) check;
(iii) if approved by the Administrator, as determined in its sole discretion, surrender of other Shares which meet the conditions established by the Administrator to avoid adverse accounting consequences to the Company (as determined by the Administrator);
(iv) if approved by the Administrator, as determined in its sole discretion, by a broker-assisted cashless exercise in accordance with procedures approved by the Administrator, whereby payment of the exercise price may be satisfied, in whole or in part, with Shares subject to the Stock Option by delivery of an irrevocable direction to a securities broker (on a form prescribed by the Administrator) to sell Shares and to deliver all or part of the sale proceeds to the Company in payment of the aggregate exercise price;
(v) if approved by the Administrator for a Nonqualified Stock Option, as determined in its sole discretion, by delivery of a notice of “net exercise” to the Company, pursuant to which the Participant shall receive the number of Shares underlying the Stock Option so exercised reduced by the number of Shares equal to the aggregate exercise price of the Stock Option divided by the Fair Market Value on the date of exercise;
(vi) such other consideration and method of payment for the issuance of Shares to the extent permitted by Applicable Laws; or
(vii) any combination of the foregoing methods of payment.
Annex J-7
(d) Exercise of Stock Option.
(i) Procedure for Exercise. Any Stock Option granted hereunder will be exercisable according to the terms of the Plan and at such times and under such conditions as determined by the Administrator and set forth in the Award Agreement. A Stock Option may not be exercised for a fraction of a Share. Exercising a Stock Option in any manner will decrease the number of Shares thereafter available for purchase under the Stock Option, by the number of Shares as to which the Stock Option is exercised.
(ii) Exercise Requirements. A Stock Option will be deemed exercised when the Company receives: (A) written or electronic notice of exercise (in accordance with the Award Agreement) from the person entitled to exercise the Stock Option, and (B) full payment of the exercise price (including provision for any applicable tax withholding).
(iii) Non-Exempt Employees. If a Stock Option is granted to an Employee who is a non-exempt employee for purposes of the Fair Labor Standards Act of 1938, as amended, the Stock Option will not be first exercisable for any Shares until at least six (6) months following the date of grant of the Stock Option (although the Stock Option may vest prior to such date). Consistent with the provisions of the Worker Economic Opportunity Act, (A) if such non-exempt Employee dies or suffers a Disability, (B) upon a Change in Control in which such Stock Option is not assumed, continued, or substituted, or (C) upon the Participant’s retirement (as such term may be defined in the Participant’s Award Agreement, in another agreement between the Participant and the Company or a Subsidiary, or, if no such definition, in accordance with the then current employment policies and guidelines of the Company or employing Subsidiary), the vested portion of any Stock Option may be exercised earlier than six (6) months following the date of grant. The foregoing provision is intended to operate so that any income derived by a non-exempt employee in connection with the exercise or vesting of a Stock Option will be exempt from the Participant’s regular rate of pay. To the extent permitted and/or required for compliance with the Worker Economic Opportunity Act to ensure that any income derived by a non-exempt employee in connection with the exercise, vesting, or issuance of any Shares under any other Award will be exempt from the employee’s regular rate of pay, the provisions of this Section 7(d)(iii) will apply to all Awards and are hereby incorporated by reference into such Award Agreements.
(iv) Termination of Relationship as a Service Provider. If a Participant ceases to be a Service Provider, the Participant may exercise the Stock Option within such period of time as is specified in the Award Agreement to the extent that the Stock Option is vested on the date of termination (but in no event later than the expiration of the term of such Stock Option as set forth in the Award Agreement). In the absence of a specified time in the Award Agreement, the Stock Option will remain exercisable for three (3) months (or twelve (12) months in the case of termination on account of Disability or death) following the Participant’s termination. If a Participant commits an act of Cause, all vested and unvested Stock Options shall be forfeited as of such date. Unless otherwise provided by the Administrator, if on the date of termination the Participant is not vested as to a Stock Option, the Shares covered by the unvested portion of the Stock Option will be forfeited and will revert to the Plan and again will become available for grant under the Plan. If after termination, the Participant does not exercise a Stock Option as to all of the vested Shares within the time specified by the Administrator, the Stock Option will terminate, and remaining Shares covered by such Stock Option will be forfeited and will revert to the Plan and again will become available for grant under the Plan.
(v) Extension of Exercisability. A Participant may not exercise a Stock Option at any time that the issuance of Shares upon such exercise would violate Applicable Laws. Except as otherwise provided in the Award Agreement, if a Participant ceases to be a Service Provider for any reason other than for Cause and, at any time during the last thirty (30) days of the applicable post-termination exercise period: (A) the exercise of the Participant’s Stock Option would be prohibited solely because the issuance of Shares upon such exercise would violate Applicable Laws, or (B) the immediate sale of any Shares issued upon such exercise would violate the Company’s trading policy, then the applicable post-termination exercise period will be extended to the last day of the calendar month that commences following the date the Award would otherwise expire, with an additional extension of the exercise period to the last day of the next calendar month to apply if any of the foregoing restrictions apply at any time during such extended exercise period, generally without limitation as to the maximum permitted number of extensions); provided, however, that in no event may such Award be exercised after the expiration of its maximum term.
Annex J-8
(vi) Beneficiary. If a Participant dies while a Service Provider, the Stock Option may be exercised following the Participant’s death by the Participant’s designated beneficiary, provided such beneficiary has been designated and received by the Administrator prior to the Participant’s death in a form acceptable to the Administrator. If no such beneficiary has been properly designated by the Participant, then such Stock Option may be exercised by the personal representative of the Participant’s estate or by the persons to whom the Stock Option is transferred pursuant to the Participant’s will or in accordance with the laws of descent and distribution.
(vii) Shareholder Rights. Until the Shares are issued (as evidenced by the appropriate entry on the books of the Company or of a duly authorized transfer agent or depositary of the Company), no right to vote or receive dividends or any other rights as a shareholder will exist with respect to the Shares, notwithstanding the exercise of the Stock Option. No adjustment will be made for a dividend or other right for which the record date is prior to the date the Shares are issued, except as provided in Section 14 or the applicable Award Agreement.
(e) Incentive Stock Option Limitations.
(i) Each Stock Option will be designated in the Award Agreement as either an Incentive Stock Option or a Nonqualified Stock Option. However, notwithstanding such designation, to the extent that the aggregate Fair Market Value of the Shares with respect to which Incentive Stock Options are exercisable for the first time by the Participant during any calendar year (under all plans of the Company, its Parent, or any Subsidiary) exceeds $100,000 (or such other limit established in the Code), such Stock Options will be treated as Nonqualified Stock Options. For purposes of this Section 7(e)(i), Incentive Stock Options will be taken into account in the order in which they were granted. The Fair Market Value of the Shares will be determined as of the time the Stock Option is granted.
(ii) In the case of an Incentive Stock Option, the term will be ten (10) years from the date of grant or such shorter term as may be provided in the Award Agreement. Moreover, in the case of an Incentive Stock Option granted to a Participant who, at the time the Incentive Stock Option is granted, owns shares representing more than ten percent (10%) of the total combined voting power of all classes of stock of the Company, its Parent, or any Subsidiary, the term of the Incentive Stock Option will be five (5) years from the date of grant or such shorter term as may be provided in the Award Agreement and the exercise price shall not be less than one hundred ten percent (110%) of the Fair Market Value per Share on the date of grant.
(iii) No Stock Option shall be treated as an Incentive Stock Option unless this Plan has been approved by the shareholders of the Company in a manner intended to comply with the shareholder approval requirements of Code Section 422(b)(1), provided that any Stock Option intended to be an Incentive Stock Option shall not fail to be effective solely on account of a failure to obtain such approval, but rather such Stock Option shall be treated as a Nonqualified Stock Option unless and until such approval is obtained.
(iv) In the case of an Incentive Stock Option, the terms and conditions of such grant shall be subject to and comply with such rules as may be prescribed by Code Section 422. If for any reason a Stock Option intended to be an Incentive Stock Option (or any portion thereof) shall not qualify as an Incentive Stock Option, then, to the extent of such nonqualification, such Stock Option or portion thereof shall be regarded as a Nonqualified Stock Option appropriately granted under this Plan.
8. Stock Appreciation Rights (“SARs”). The Administrator, at any time and from time to time, may grant SARs to Service Providers. Each Award of SARs shall be subject to such terms and conditions, consistent with the Plan, as the Administrator may impose from time to time, subject to the following limitations:
(a) SAR Award Agreement. Each SAR will be evidenced by an Award Agreement that will specify the exercise price, the term of the SAR, the conditions of exercise, and such other terms and conditions as the Administrator, in its sole discretion, will determine.
(b) Number of Shares. The Administrator will have complete discretion to determine the number of Shares subject to any SAR.
Annex J-9
(c) Exercise Price and Other Terms. The per share exercise price for the Shares that will determine the amount of the payment to be received upon exercise of a SAR will be determined by the Administrator and will be no less than one hundred percent (100%) of the Fair Market Value per Share on the date of grant. Notwithstanding the foregoing, in the case of a SAR that is a Substitute Award, the exercise price for Shares subject to such SAR may be less than the Fair Market Value per Share on the date of grant; provided that the exercise price of any Substitute Award shall be determined in accordance with the applicable requirements of Code Sections 424 and 409A. Otherwise, the Administrator, subject to the provisions of the Plan, will have complete discretion to determine the terms and conditions of any SAR.
(d) Expiration of Stock Appreciation Rights. A SAR will expire upon the date determined by the Administrator, in its sole discretion, and set forth in the Award Agreement. Notwithstanding the foregoing, the rules of Section 7(d) relating to the maximum term and exercise price also will apply to SARs issued in tandem with an Incentive Stock Option.
(e) Payment of Stock Appreciation Right Amount. Upon exercise of a SAR, a Participant will be entitled to receive payment from the Company in an amount determined by multiplying:
(i) the difference between the Fair Market Value of a Share on the date of exercise over the exercise price; times
(ii) the number of Shares with respect to which the SAR is exercised.
(f) Payment Form. At the discretion of the Administrator, the payment upon SAR exercise may be in cash, in Shares, other securities, or other property of equivalent value, or in some combination thereof.
(g) Tandem Awards. Any Stock Option granted under this Plan may include tandem SARs (i.e., SARs granted in conjunction with an Award of Stock Options under this Plan). The Administrator also may award SARs to a Service Provider independent of any Stock Option.
9. Restricted Stock. The Administrator, at any time and from time to time, may grant Restricted Stock to Service Providers in such amounts as the Administrator, in its sole discretion, will determine, subject to the following limitations:
(a) Restricted Stock Agreement. Each Award of Restricted Stock will be evidenced by an Award Agreement that will specify the Period of Restriction and the applicable restrictions, the number of Shares granted, and such other terms and conditions as the Administrator, in its sole discretion, will determine. Restricted Stock may be awarded in consideration for (i) cash, check, bank draft or money order payable to the Company, (ii) past Service, or (iii) any other form of legal consideration (including future Service) that may be acceptable to the Administrator, in its sole discretion, and permissible under Applicable Laws.
(b) Removal of Restrictions. Unless the Administrator determines otherwise, Restricted Stock will be held by the Company as escrow agent until the restrictions on such Restricted Stock have lapsed. The Administrator, in its discretion, may accelerate the time at which any restrictions will lapse or be removed.
(c) Voting Rights. During the Period of Restriction, a Participant holding Restricted Stock may exercise the voting rights applicable to those restricted Shares, unless the Administrator determines otherwise.
(d) Dividends and Other Distributions. Except as provided in the Award Agreement, during the Period of Restriction, a Participant holding Restricted Stock will be entitled to receive all dividends and other distributions paid with respect to such Restricted Stock. If any such dividends or distributions are paid in Shares, such Shares will be subject to the same restrictions on transferability and forfeitability as the Restricted Stock with respect to which they were paid.
(e) Transferability. Restricted Stock may not be sold, transferred, pledged, assigned, or otherwise alienated or hypothecated until the end of the applicable Period of Restriction.
(f) Return of Restricted Stock to Company. On the date set forth in the Award Agreement, the Restricted Stock for which restrictions have not lapsed will be forfeited and will revert to the Company and again will become available for grant under the Plan.
Annex J-10
10. Restricted Stock Units (“RSUs”). The Administrator, at any time and from time to time, may grant RSUs under the Plan to Service Providers. Each RSU shall be subject to such terms and conditions, consistent with the Plan, as the Administrator may impose from time to time, subject to the following limitations:
(a) RSU Award Agreement. Each Award of RSUs will be evidenced by an Award Agreement that will specify the terms, conditions, and restrictions related to the grant, including the number of RSUs and such other terms and conditions as the Administrator, in its sole discretion, will determine.
(b) Vesting Criteria and Other Terms. The Administrator will set vesting criteria in its discretion, which, depending on the extent to which the criteria are met, will determine the number of RSUs that will be paid out to the Participant. The Administrator may set vesting criteria based upon the achievement of Company-wide, business unit, or individual goals (including, but not limited to, continued employment or Service), or any other basis determined by the Administrator in its discretion.
(c) Earning Restricted Stock Units. Upon meeting the applicable vesting criteria, the Participant will be entitled to receive a payout as determined by the Administrator. Notwithstanding the foregoing, at any time after the grant of RSUs, the Administrator, in its sole discretion, may reduce or waive any vesting criteria that must be met to receive a payout.
(d) Form and Timing of Payment. Payment of earned RSUs will be made as soon as practicable after the date(s) determined by the Administrator and set forth in the Award Agreement. The Administrator, in its sole discretion, may settle earned RSUs in cash, Shares, other securities, other property, or a combination of both.
(e) Voting and Dividend Equivalent Rights. The holders of RSUs shall have no voting rights as the Company’s shareholders. Prior to settlement or forfeiture, RSUs awarded under the Plan may, at the Administrator’s discretion, provide for a right to dividend equivalents. Such right entitles the holder to be credited with an amount equal to all dividends paid on one Share while the RSU is outstanding. Dividend equivalents may be converted into additional RSUs. Settlement of dividend equivalents may be made in the form of cash, Shares, other securities, other property, or in a combination of the foregoing. Prior to distribution, any dividend equivalents shall be subject to the same conditions and restrictions as the RSUs to which they attach.
(f) Cancellation. On the date set forth in the Award Agreement, all unearned RSUs will be forfeited to the Company.
11. Other Stock-Based Awards. Other Stock-Based Awards may be granted either alone, in addition to, or in tandem with, other Awards granted under the Plan and/or cash awards made outside of the Plan. The Administrator shall have authority to determine the Service Providers to whom and the time or times at which Other Stock-Based Awards shall be made, the amount of such Other Stock-Based Awards, and all other conditions of the Other Stock-Based Awards including any dividend and/or voting rights.
12. Vesting.
(a) Vesting Conditions. Each Award may or may not be subject to vesting, a Period of Restriction, and/or other conditions as the Administrator may determine. Vesting shall occur, in full or in installments, upon satisfaction of the conditions specified in the Award Agreement. Vesting conditions may include Service-based conditions, performance-based conditions, such other conditions as the Administrator may determine, or any combination thereof. Unless specifically set forth in the Award Agreement, Awards shall not be considered subject to any performance-based condition. An Award Agreement may provide for accelerated vesting upon certain specified events.
(b) Performance Criteria. The Administrator may establish performance-based conditions for an Award as specified in the Award Agreement, which may be based on the attainment of specific levels of performance of the Company (and/or one or more Subsidiaries, divisions, business segments or operational units, or any combination of the foregoing) and may include, without limitation, any of the following: (i) net earnings or net income (before or after taxes); (ii) basic or diluted earnings per share (before or after taxes); (iii) revenue or revenue growth (measured on a net or gross basis); (iv) gross profit or gross profit growth; (v) operating profit (before or after taxes); (vi) return measures (including, but not limited to, return on assets, capital, invested capital, equity, or sales); (vii) cash flow (including, but not limited to, operating cash flow, free
Annex J-11
cash flow, net cash provided by operations and cash flow return on capital); (viii) financing and other capital raising transactions (including, but not limited to, sales of the Company’s equity or debt securities); (ix) earnings before or after taxes, interest, depreciation and/or amortization; (x) gross or operating margins; (xi) productivity ratios; (xii) share price (including, but not limited to, growth measures and total shareholder return); (xiii) expense targets; (xiv) margins; (xv) productivity and operating efficiencies; (xvi) customer satisfaction; (xvii) customer growth; (xviii) working capital targets; (xix) measures of economic value added; (xx) inventory control; (xxi) enterprise value; (xxii) sales; (xxiii) debt levels and net debt; (xxiv) combined ratio; (xxv) timely launch of new facilities; (xxvi) client retention; (xxvii) employee retention; (xxviii) timely completion of new product rollouts; (xxix) cost targets; (xxx) reductions and savings; (xxxi) productivity and efficiencies; (xxxii) strategic partnerships or transactions; and (xxxiii) personal targets, goals or completion of projects. Any one or more of the performance criteria may be used on an absolute or relative basis to measure the performance of the Company and/or one or more Subsidiaries as a whole or any business unit(s) of the Company and/or one or more Subsidiaries or any combination thereof, as the Administrator may deem appropriate, or any of the above performance criteria may be compared to the performance of a selected group of comparison or peer companies, or a published or special index that the Administrator, in its sole discretion, deems appropriate, or as compared to various stock market indices. The Administrator also has the authority to provide for accelerated vesting of any Award based on the achievement of performance criteria specified in this paragraph. Any performance criteria that are financial metrics, may be determined in accordance with United States Generally Accepted Accounting Principles (“GAAP”) or may be adjusted when established to include or exclude any items otherwise includable or excludable under GAAP.
(c) Default Vesting. Unless otherwise set forth in an individual Award Agreement, each Award shall vest over a three (3) year period, with one-third (1/3) of the Award vesting on the first annual anniversary of the date of grant and the remaining portion vesting monthly thereafter.
(d) Leaves of Absence. Unless the Administrator provides otherwise, vesting of Awards granted hereunder will be suspended during any Employee’s unpaid leave of absence and will resume on the date the Employee returns to work on a regular schedule as determined by the Administrator; provided, however, that no vesting credit will be awarded for the time vesting has been suspended during such leave of absence. A Service Provider will not cease to be an Employee in the case of (i) any leave of absence approved by the Company or the employing Subsidiary, although any leave of absence not provided for in the applicable employee manual of the Company or employing Subsidiary needs to be approved by the Administrator, or (ii) transfers between locations of the Company or between the Company, its Parent, or any Subsidiary. For purposes of Incentive Stock Options, no leave of absence may exceed ninety (90) days, unless reemployment upon expiration of such leave is guaranteed by statute or contract. If reemployment upon expiration of a leave of absence approved by the Company or employing Subsidiary is not so guaranteed, then three (3) months following the 91st day of such leave any Incentive Stock Option held by the Participant will cease to be treated as an Incentive Stock Option and will be treated for federal tax purposes as a Nonqualified Stock Option.
(e) Change in Status. In the event a Service Provider’s regular level of time commitment in the performance of Services is reduced (for example, and without limitation, if the Service Provider is an Employee of the Company and the Employee has a change in status from a full-time Employee to a part-time Employee) after the date of grant of any Award to the Service Provider, the Administrator has the right in its sole discretion to (i) make a corresponding reduction in the number of Shares subject to any portion of such Award that is scheduled to vest or become payable after the date of such change in time commitment, and (ii) in lieu of or in combination with such a reduction, extend the vesting or payment schedule applicable to such Award. In the event of any such reduction, the Service Provider will have no right with respect to any portion of the Award that is so reduced or extended.
13. Non-Transferability of Awards. Unless determined otherwise by the Administrator, an Award may not be sold, pledged, assigned, hypothecated, transferred, or disposed of in any manner, except to the Participant’s estate or legal representative, and may be exercised, during the lifetime of the Participant, only by the Participant, although the Administrator, in its discretion, may permit Award transfers for purposes of estate planning or charitable giving. If the Administrator makes an Award transferable, such Award will contain such additional terms and conditions as the Administrator deems appropriate.
Annex J-12
14. Adjustments; Dissolution or Liquidation; Change in Control.
(a) Adjustments. In the event that any dividend or other distribution (whether in the form of cash, Shares, other securities, or other property), recapitalization, share split, reverse share split, reorganization, merger, consolidation, split-up, spin-off, combination, repurchase, or exchange of Shares or other securities of the Company, or other change in the corporate structure of the Company affecting the Shares occurs such that an adjustment is determined by the Administrator (in its sole discretion) to be appropriate in order to prevent dilution or enlargement of the benefits or potential benefits intended to be made available under the Plan, then the Administrator shall, in such manner as it may deem equitable, adjust (i) the number and class of Shares which may be delivered under the Plan; (ii) the number, class and price of Shares subject to outstanding Awards, and (iii) the numerical limits in Section 4. Notwithstanding the preceding, the number of Shares subject to any Award always shall be a whole number.
(b) Dissolution or Liquidation. In the event of the proposed dissolution or liquidation of the Company, the Administrator will notify each Participant as soon as practicable prior to the effective date of such proposed transaction. The Administrator in its discretion may provide for a Participant to have the right to exercise an Award, to the extent applicable, until ten (10) days prior to such transaction as to all of the Shares covered thereby, including Shares as to which the Award would not be vested or otherwise be exercisable. In addition, the Administrator may provide that any Company repurchase option or forfeiture rights applicable to any Award shall lapse one hundred percent (100%), and that any Award vesting shall accelerate one hundred percent (100%), provided the proposed dissolution or liquidation takes place at the time and in the manner contemplated. To the extent it has not been previously vested and, if applicable, exercised, an Award will terminate immediately prior to the consummation of such proposed action.
(c) Change in Control.
(i) In the event of a Change in Control, each outstanding Award shall be assumed or an equivalent award substituted by the acquiring or successor corporation or a parent of the acquiring or successor corporation.
(ii) Unless determined otherwise by the Administrator, in the event that the successor corporation refuses to assume or substitute for the Award, (A) the Participant shall fully vest in and have the right to exercise the Award as to all of the Shares, including those as to which it would not otherwise be vested or exercisable; (B) all applicable restrictions will lapse; and (C) all performance objectives and other vesting criteria will be deemed achieved at targeted levels.
(iii) If a Stock Option or SAR is not assumed or substituted in the event of a Change in Control, the Administrator shall notify the Participant in writing or electronically that the Stock Option or SAR shall be exercisable, to the extent vested, for a period of up to fifteen (15) days from the date of such notice, and the Stock Option or SAR shall terminate upon the expiration of such period.
(iv) For the purposes of this Section 14, the Award shall be considered assumed if, following the Change in Control, the Award confers the right to purchase or receive, for each Share subject to the Award immediately prior to the Change in Control, the consideration (whether shares, cash, or other securities or property) received in the Change in Control by holders of Shares for each Share held on the effective date of the transaction (and if holders were offered a choice of consideration, the type of consideration chosen by the holders of a majority of the outstanding Shares); provided, however, that if such consideration received in the Change in Control is not solely common shares of the acquiring or successor corporation or its parent, the Administrator may, with the consent of the acquiring or successor corporation, provide for the consideration to be received, for each Share subject to the Award, to be solely common shares of the acquiring or successor corporation or its parent equal in fair market value to the per share consideration received by holders of Shares in the Change in Control.
(v) Notwithstanding anything herein to the contrary, an Award that vests, is earned, or is paid out upon the satisfaction of one or more performance goals will not be considered assumed if the Company or the acquiring or successor corporation modifies any of such performance goals without the Participant’s consent; provided, however, that a modification to such performance goals only to reflect the acquiring or successor corporation’s post-Change in Control corporate structure will not be deemed to invalidate an otherwise valid Award assumption.
Annex J-13
(vi) Payments under this Section 14 may be delayed to the same extent that payment of consideration to the holders of Shares in connection with the Change in Control is delayed as a result of escrows, earn outs, holdbacks, or any other contingencies.
15. Taxes.
(a) General. It is a condition to each Award under the Plan that a Participant or such Participant’s successor shall make such arrangements that may be necessary, in the opinion of the Administrator or the Company, for the satisfaction of any federal, state, local, or foreign withholding tax obligations that arise in connection with any Award. The Company shall not be required to issue any Shares or make any cash payment under the Plan unless such obligations are satisfied.
(b) Share Withholding. To the extent that Applicable Laws subject a Participant to tax withholding obligations, the Administrator may permit such Participant to satisfy all or part of such obligations by having the Company, its Parent, or any Subsidiary withhold all or a portion of any Share that otherwise would be issued to such Participant or by surrendering all or a portion of any Share that the Participant previously acquired. Such Share shall be valued on the date withheld or surrendered. Any payment of taxes by assigning Shares to the Company, its Parent, or any Subsidiary may be subject to restrictions, including any restrictions required by the Securities and Exchange Commission, accounting, or other rules.
(c) Discretionary Nature of Plan. The benefits and rights provided under the Plan are wholly discretionary and, although provided by the Company, do not constitute regular or periodic payments. Unless otherwise required by Applicable Laws, the benefits and rights provided under the Plan are not to be considered part of a Participant’s salary or compensation or for purposes of calculating any severance, resignation, redundancy, other end of service payments, vacation, bonuses, long-term service awards, indemnification, pension or retirement benefits, or any other payments, benefits, or rights of any kind. By acceptance of an Award, a Participant waives any and all rights to compensation or damages as a result of the termination of Service for any reason whatsoever insofar as those rights result or may result from this Plan or any Award.
16. No Rights as a Service Provider. Neither the Plan, nor an Award Agreement, nor any Award shall confer upon a Participant any right with respect to continuing a relationship as a Service Provider, nor shall they interfere in any way with the right of the Participant or the right of the Company, its Parent, or any Subsidiary to terminate such relationship at any time, with or without cause.
17. Recoupment Policy. All Awards granted under the Plan, all amounts paid under the Plan, and all Shares issued under the Plan shall be subject to reduction, recoupment, clawback, or recovery by the Company in accordance with Applicable Laws and with Company policy (whenever adopted) regarding same, whether or not such policy is intended to satisfy the requirements of the Dodd-Frank Wall Street Reform and Consumer Protection Act, the Sarbanes-Oxley Act, or other Applicable Laws, as well as any implementing regulations and/or listing standards.
18. Amendment and Termination of the Plan.
(a) Amendment and Termination. The Board may at any time amend, alter, suspend, or terminate the Plan.
(b) Shareholder Approval. The Company may obtain shareholder approval of any Plan amendment to the extent necessary or, as determined by the Administrator in its sole discretion, desirable to comply with Applicable Laws, including any amendment that (i) increases the number of Shares available for issuance under the Plan or (ii) changes the persons or class of persons eligible to receive Awards.
(c) Effect of Amendment or Termination. No amendment, alteration, suspension, or termination of the Plan will materially impair the rights of any Participant with respect to outstanding Awards, unless mutually agreed otherwise between the Participant and the Administrator, which agreement must be in writing and signed by the Participant and the Company. Termination of the Plan will not affect the Administrator’s ability to exercise the powers granted to it hereunder with respect to Awards granted under the Plan prior to the date of such termination.
Annex J-14
19. Conditions Upon Issuance of Shares.
(a) Legal Compliance. Shares will not be issued pursuant to an Award unless the exercise of such Award and the issuance and delivery of such Shares will comply with Applicable Laws and will be further subject to the approval of counsel for the Company with respect to such compliance.
(b) Investment Representations. As a condition to the exercise or receipt of an Award, the Company may require the person exercising or receiving such Award to represent and warrant at the time of any such exercise or receipt that the Shares are being purchased only for investment and without any present intention to sell or distribute such Shares if, in the opinion of counsel for the Company, such a representation is required or desirable.
20. Severability. Notwithstanding any contrary provision of the Plan or an Award Agreement, if any one or more of the provisions (or any part thereof) of this Plan or an Award Agreement shall be held invalid, illegal, or unenforceable in any respect, such provision shall be modified so as to make it valid, legal, and enforceable, and the validity, legality, and enforceability of the remaining provisions (or any part thereof) of the Plan or Award Agreement, as applicable, shall not in any way be affected or impaired thereby.
21. Inability to Obtain Authority. The inability of the Company to obtain authority from any regulatory body having jurisdiction, which authority is deemed by the Company’s counsel to be necessary to the lawful issuance and sale of any Shares hereunder, will relieve the Company of any liability in respect of the failure to issue or sell such Shares as to which such requisite authority will not have been obtained.
22. Shareholder Approval. The Plan will be subject to approval by the shareholders of the Company within twelve (12) months after the date the Plan is adopted. Such shareholder approval will be obtained in the manner and to the degree required under Applicable Laws. All Awards hereunder are contingent on approval of the Plan by shareholders. Notwithstanding any other provision of this Plan, if the Plan is not approved by the shareholders within twelve (12) months after the date the Plan is adopted, the Plan and any Awards hereunder shall be automatically terminated.
23. Choice of Law. The Plan will be governed by and construed in accordance with the internal laws of the State of Delaware, without reference to any choice of law principles.
24. Effective Date.
(a) The Plan shall be effective as of ___________ _____, 2025, the date on which the Plan was adopted by the Board and the Company’s shareholders (the “Effective Date”).
(b) Unless terminated earlier under Section 18, this Plan shall terminate on ___________ _____, 2035, ten years after the Effective Date.
Annex J-15
Amended and Restated
Certificate of Incorporation
of
Apimeds Pharmaceuticals US, Inc.
December 5, 2023
Apimeds Pharmaceuticals US, Inc., a corporation organized and existing under the laws of the State of Delaware (the “Corporation”), does hereby certify as follows:
1. The name of the Corporation is Apimeds Pharmaceuticals US, Inc. The original certificate of incorporation of the Corporation was filed with the Secretary of State of the State of Delaware on May 11, 2020 (the “Certificate”). The Certificate was amended on January 6, 2022 to increase the number of the authorized shares of common stock to 15,000,000.
2. This Amended and Restated Certificate of Incorporation (the “Amended and Restated Certificate”), which both restates and amends the provisions of the Certificate, was duly adopted in accordance with Sections 228, 242 and 245 of the General Corporation Law of the State of Delaware, as amended from time to time (the “DGCL”).
3. This Amended and Restated Certificate shall become effective on the date of filing with Secretary of State of Delaware.
4. The text of the Certificate, as amended to the date hereof, is hereby restated and amended in its entirety to read as follows:
FIRST: The name of the corporation is Apimeds Pharmaceuticals US, Inc. (the “Corporation”).
SECOND: The address of the Corporation’s registered office in the State of Delaware is c/o Corporation Service Company, 251 Little Falls Drive, County of New Castle, Wilmington, Delaware 19808. The name of the registered agent is Corporation Service Company.
THIRD: The purpose of the Corporation is to engage in any lawful act or activity for which Corporations may be organized under the General Corporation Law of the State of Delaware.
FOURTH: The aggregate number of shares of all classes of capital stock that the Corporation shall have the authority to issue is 110,00,000, consisting of (i) 100,000,000 shares of common stock, par value $0.01 per share (the “Common Stock”); and (ii) 10,000,000 shares of Preferred Stock, par value $0.01 per share (the “Preferred Stock”).
FIFTH: The Board of Directors of the Corporation is expressly authorized and empowered to adopt, amend or repeal the by-laws of the Corporation, without any action on the part of the stockholders of the Corporation, but the stockholders of the Corporation may make additional by-laws and may amend or repeal any by-laws whether adopted by them or otherwise.
SIXTH: Whenever a compromise or arrangement is proposed between the Corporation and its creditors or any class of them and/or between this Corporation and its stockholders or any class of them, any court of equitable jurisdiction within the State of Delaware may, on the application in a summary way of this Corporation or of any creditor or stockholder thereof or on the application of any receiver or receivers appointed for this Corporation under the provisions of Section 291 of Title 8 of the Delaware Code or on the application of trustees in dissolution or of any receiver or receivers appointed for this Corporation under the provisions of Section 279 of Title 8 of the Delaware Code, order a meeting of the creditors or class of creditors, and/or of the stockholders or class of stockholders of this Corporation, as the case may be, to be summoned in such manner as the said court directs. If a majority in number representing three-fourths in value of the creditors or class of creditors, and/or of the stockholders or class of stockholders of this Corporation, as the case may be, agree to any compromise or arrangement and to any reorganization of this Corporation as a consequence of such compromise or arrangement, the said compromise or arrangement and the said reorganization shall, if sanctioned by the court to which the said application has been made, be binding on all the creditors or class of creditors, and/or on all the stockholders or class of stockholders, of this Corporation, as the case may be, and also of this Corporation.
Annex K-1
SEVENTH: The personal liability of the directors of the Corporation is hereby eliminated to the fullest extent permitted by subsection 102(b)(7) of the General Corporation Law of the State of Delaware, as the same may be amended and supplemented.
EIGHTH: The Corporation shall, to the fullest extent permitted by Section 145 of the General Corporation Law of the State of Delaware, as the same may be amended and supplemented, indemnify any and all persons whom it shall have power to indemnify under said section from and against any and all of the expenses, liabilities, or other matters referred to in or covered by said section, and the indemnification provided for herein shall not be deemed exclusive of any other rights to which those indemnified may be entitled under any by-law, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in his official capacity and as to action in another capacity while holding such office, and shall continue as to a person who has ceased to be a director, officer, employee, or agent and shall inure to the benefit of the heirs, executors, and administrators of such a person.
Annex K-2
IN WITNESS WHEREOF, this Amended and Restated Certificate of Incorporation has been executed by a duly authorized officer of this Corporation on this 4th day of December, 2023.
|
Apimeds Pharmaceuticals US, Inc. |
||||
|
/s/ Erik Emerson |
||||
|
Name: |
Erik Emerson |
|||
|
Title: |
Chief Executive Officer |
|||
Annex K-3
AMENDED AND RESTATED
BYLAWS
OF
APIMEDS PHARMACEUTICALS US, INC.
ARTICLE I
OFFICES
Section 1. Registered Office. The registered office of the corporation in the State of Delaware shall be at 251 Little Falls Drive, City of Wilmington, County of New Castle, Delaware 19801.
The name of the registered agent at such address is Corporation Service Company, 251 Little Falls Drive, City of Wilmington, County of New Castle, Delaware 19801.
Section 2. Other Offices. The corporation shall also have and maintain an office or principal place of business at such place as may be fixed by the corporation’s Board of Directors (the “Board of Directors”), and may also have offices at such other places, both within and without the State of Delaware as the Board of Directors may from time to time determine or the business of the corporation may require.
ARTICLE II
CORPORATE SEAL
Section 3. Corporate Seal. The Board of Directors may adopt a corporate seal. The corporate seal shall consist of a die bearing the name of the corporation and the inscription, “Corporate Seal-Delaware.” Said seal may be used by causing it or a facsimile thereof to be impressed or affixed or reproduced or otherwise.
ARTICLE III
STOCKHOLDERS’ MEETINGS
Section 4. Place of Meetings. Meetings of the stockholders of the corporation may be held at such place, either within or without the State of Delaware, as may be determined from time to time by the Board of Directors. The Board of Directors may, in its sole discretion, determine that the meeting shall not be held at any place, but may instead be held solely by means of remote communication as provided under the Delaware General Corporation Law (the “DGCL”).
Section 5. Annual Meetings.
(a) The annual meeting of the stockholders of the corporation, for the purpose of election of directors and for such other business as may properly come before it, shall be held on such date and at such time as may be designated from time to time by the Board of Directors. Nominations of persons for election to the Board of Directors of the corporation and the proposal of business to be considered by the stockholders may be made at an annual meeting of stockholders: (i) pursuant to the corporation’s notice of meeting of stockholders (with respect to business other than nominations); (ii) brought specifically by or at the direction of the Board of Directors; or (iii) by any stockholder of the corporation who was a stockholder of record at the time of giving the stockholder’s notice provided for in Section 5(b) of these Amended and Restated Bylaws (the “Bylaws”), who is entitled to vote at the meeting and who complied with the notice procedures set forth in this Section 5. For the avoidance of doubt, clause (iii) above shall be the exclusive means for a stockholder to make nominations and submit other business (other than matters properly included in the corporation’s notice of meeting of stockholders and proxy statement under Rule 14a-8 under the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder (the “1934 Act”)) before an annual meeting of stockholders.
(b) At an annual meeting of the stockholders, only such business shall be conducted as is a proper matter for stockholder action under Delaware law and as shall have been properly brought before the meeting.
(i) For nominations for the election to the Board of Directors to be properly brought before an annual meeting by a stockholder pursuant to clause (iii) of Section 5(a) of these Bylaws, the stockholder must deliver written notice to the Secretary at the principal executive offices of the corporation on a timely basis as set forth in
Annex L-1
Section 5(b)(iii) of these Bylaws and must update and supplement such written notice on a timely basis as set forth in Section 5(c) of these Bylaws. Such stockholder’s notice shall set forth: (A) as to each nominee such stockholder proposes to nominate at the meeting: (1) the name, age, business address and residence address of such nominee; (2) the principal occupation or employment of such nominee; (3) the class and number of shares of each class of capital stock of the corporation which are owned of record and beneficially by such nominee; (4) the date or dates on which such shares were acquired and the investment intent of such acquisition; (5) with respect to each nominee for election or re-election to the Board of Directors, include a completed and signed questionnaire, representation and agreement required by Section 5(e) of these Bylaws; and (6) such other information concerning such nominee as would be required to be disclosed in a proxy statement soliciting proxies for the election of such nominee as a director in an election contest (even if an election contest is not involved), or that is otherwise required to be disclosed pursuant to Section 14 of the 1934 Act and the rules and regulations promulgated thereunder (including such person’s written consent to being named as a nominee and to serving as a director if elected); and (B) the information required by Section 5(b)(iv) of these Bylaws. The corporation may require any proposed nominee to furnish such other information as it may reasonably require to determine the eligibility of such proposed nominee to serve as an independent director of the corporation or that could be material to a reasonable stockholder’s understanding of the independence, or lack thereof, of such proposed nominee.
(ii) Other than proposals sought to be included in the corporation’s proxy materials pursuant to Rule 14(a)-8 under the 1934 Act, for business other than nominations for the election to the Board of Directors to be properly brought before an annual meeting by a stockholder pursuant to clause (iii) of Section 5(a) of these Bylaws, the stockholder must deliver written notice to the Secretary at the principal executive offices of the corporation on a timely basis as set forth in Section 5(b)(iii) of these Bylaws, and must update and supplement such written notice on a timely basis as set forth in Section 5(c) of these Bylaws. Such stockholder’s notice shall set forth: (A) as to each matter such stockholder proposes to bring before the meeting, a brief description of the business desired to be brought before the meeting, the reasons for conducting such business at the meeting, and any material interest (including any anticipated benefit of such business to any Proponent (as defined below) other than solely as a result of its ownership of the corporation’s capital stock, that is material to any Proponent individually, or to the Proponents in the aggregate) in such business of any Proponent; and (B) the information required by Section 5(b)(iv) of these Bylaws.
(iii) To be timely, the written notice required by Section 5(b)(i) or 5(b)(ii) of these Bylaws must be received by the Secretary at the principal executive offices of the corporation not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the first anniversary of the preceding year’s annual meeting; provided, however, that, subject to the last sentence of this Section 5(b)(iii), in the event that the date of the annual meeting is advanced more than 30 days prior to or delayed by more than 30 days after the anniversary of the preceding year’s annual meeting, notice by the stockholder to be timely must be so received not earlier than the close of business on the 120th day prior to such annual meeting and not later than the close of business on the later of the 90th day prior to such annual meeting or the 10th day following the day on which public announcement of the date of such meeting is first made. In no event shall an adjournment or a postponement of an annual meeting for which notice has been given, or the public announcement thereof has been made, commence a new time period for the giving of a stockholder’s notice as described above.
(iv) The written notice required by Section 5(b)(i) or 5(b)(ii) of these Bylaws shall also set forth, as of the date of the notice and as to the stockholder giving the notice and the beneficial owner, if any, on whose behalf the nomination or proposal is made (each, a “Proponent” and collectively, the “Proponents”): (A) the name and address of each Proponent, as they appear on the corporation’s books; (B) the class, series and number of shares of the corporation that are owned beneficially and of record by each Proponent; (C) a description of any agreement, arrangement or understanding (whether oral or in writing) with respect to such nomination or proposal between or among any Proponent and any of its affiliates or associates, and any others (including their names) acting in concert, or otherwise under the agreement, arrangement or understanding, with any of the foregoing; (D) a representation that the Proponents are holders of record or beneficial owners, as the case may be, of shares of the corporation entitled to vote at the meeting and intend to appear in person or by proxy at the meeting to nominate the person or persons specified in the notice (with respect to a notice under Section 5(b)(i) of these Bylaws) or to propose the business that is specified in the notice (with respect to a notice under Section 5(b)(ii) of these Bylaws); (E) a representation as to whether the Proponents intend to deliver a proxy statement and form of proxy to holders of a sufficient number of holders of the corporation’s voting shares to elect such nominee or nominees (with respect to a notice under Section 5(b)(i) of these Bylaws) or to carry such proposal (with respect to a notice under Section 5(b)(ii) of these Bylaws); (F) to the extent known by any Proponent, the name and address of any other stockholder supporting the proposal on the date of such
Annex L-2
stockholder’s notice; and (G) a description of all Derivative Transactions (as defined below) by each Proponent during the previous 12 month period, including the date of the transactions and the class, series and number of securities involved in, and the material economic terms of, such Derivative Transactions.
For purposes of Sections 5 and 6 of these Bylaws, a “Derivative Transaction” means any agreement, arrangement, interest or understanding entered into by, or on behalf or for the benefit of, any Proponent or any of its affiliates or associates, whether record or beneficial:
(w) the value of which is derived in whole or in part from the value of any class or series of shares or other securities of the corporation;
(x) which otherwise provides any direct or indirect opportunity to gain or share in any gain derived from a change in the value of securities of the corporation;
(y) the effect or intent of which is to mitigate loss, manage risk or benefit of security value or price changes; or
(z) which provides the right to vote or increase or decrease the voting power of, such Proponent, or any of its affiliates or associates, with respect to any securities of the corporation,
which agreement, arrangement, interest or understanding may include, without limitation, any option, warrant, debt position, note, bond, convertible security, swap, stock appreciation right, short position, profit interest, hedge, right to dividends, voting agreement, performance-related fee or arrangement to borrow or lend shares (whether or not subject to payment, settlement, exercise or conversion in any such class or series), and any proportionate interest of such Proponent in the securities of the corporation held by any general or limited partnership, or any limited liability company, of which such Proponent is, directly or indirectly, a general partner or managing member.
(c) A stockholder providing written notice required by Section 5(b)(i) or (ii) of these Bylaws shall update and supplement such notice in writing, if necessary, so that the information provided or required to be provided in such notice is true and correct in all material respects as of (i) the record date for the meeting and (ii) the date that is five business days prior to the meeting and, in the event of any adjournment or postponement thereof, five business days prior to such adjourned or postponed meeting. In the case of an update and supplement pursuant to clause (i) of this Section 5(c), such update and supplement shall be received by the Secretary at the principal executive offices of the corporation not later than five business days after the record date for the meeting. In the case of an update and supplement pursuant to clause (ii) of this Section 5(c), such update and supplement shall be received by the Secretary at the principal executive offices of the corporation not later than two business days prior to the date for the meeting, and, in the event of any adjournment or postponement thereof, two business days prior to such adjourned or postponed meeting.
(d) Notwithstanding anything in Section 5(b)(iii) of these Bylaws to the contrary, in the event that the number of directors is increased and there is no public announcement of the appointment of a director, or, if no appointment was made, of the vacancy, made by the corporation at least 10 days before the last day a stockholder may deliver a notice of nomination in accordance with Section 5(b)(iii) of these Bylaws, a stockholder’s notice required by this Section 5 and which complies with the requirements in Section 5(b)(i) of these Bylaws, other than the timing requirements in Section 5(b)(iii) of these Bylaws, shall also be considered timely, but only with respect to nominees for any new positions created by such increase, if it shall be received by the Secretary at the principal executive offices of the corporation not later than the close of business on the 10th day following the day on which such public announcement is first made by the corporation.
(e) To be eligible to be a nominee for election or re-election as a director of the corporation pursuant to a nomination under clause (iii) of Section 5(a) of these Bylaws, such proposed nominee or a person on such proposed nominee’s behalf must deliver (in accordance with the time periods prescribed for delivery of notice under Section 5(b)(iii) or 5(d) of these Bylaws, as applicable) to the Secretary at the principal executive offices of the corporation a written questionnaire with respect to the background and qualification of such proposed nominee and the background of any other person or entity on whose behalf the nomination is being made (which questionnaire shall be provided by the Secretary upon written request) and a written representation and agreement (in the form provided by the Secretary upon written request) that such person (i) is not and will not become a party to (A) any agreement, arrangement or understanding with, and has not given any commitment or assurance to, any person or entity as to how such person, if elected as a director of the corporation, will act or vote on any issue or question (a “Voting Commitment”) that has not been disclosed to the corporation in the questionnaire or (B) any Voting Commitment
Annex L-3
that could limit or interfere with such person’s ability to comply, if elected as a director of the corporation, with such person’s fiduciary duties under applicable law; (ii) is not and will not become a party to any agreement, arrangement or understanding with any person or entity other than the corporation with respect to any direct or indirect compensation, reimbursement or indemnification in connection with service or action as a director of the corporation that has not been disclosed therein; and (iii) in such person’s individual capacity and on behalf of any person or entity on whose behalf the nomination is being made, would be in compliance, if elected as a director of the corporation, and will comply with, all applicable publicly disclosed corporate governance, conflict of interest, confidentiality and stock ownership and trading policies and guidelines of the corporation.
(f) A person shall not be eligible for election or re-election as a director unless the person is nominated either in accordance with clause (ii) of Section 5(a) of these Bylaws, or in accordance with clause (iii) of Section 5(a) of these Bylaws. Except as otherwise required by law, the chairman of the meeting shall have the power and duty to determine whether a nomination or any business proposed to be brought before the meeting was made, or proposed, as the case may be, in accordance with the procedures set forth in these Bylaws and, if any proposed nomination or business is not in compliance with these Bylaws, or the Proponent does not act in accordance with the representations in Sections 5(b)(iv)(D) and 5(b)(iv)(E) of these Bylaws, to declare that such proposal or nomination shall not be presented for stockholder action at the meeting and shall be disregarded, notwithstanding that proxies in respect of such nominations or such business may have been solicited or received.
(g) Notwithstanding the foregoing provisions of this Section 5, in order to include information with respect to a stockholder proposal in the proxy statement and form of proxy for a stockholders’ meeting, a stockholder must also comply with all applicable requirements of the 1934 Act and the rules and regulations thereunder. Nothing in these Bylaws shall be deemed to affect any rights of stockholders to request inclusion of proposals in the corporation’s proxy statement pursuant to Rule 14a-8 under the 1934 Act; provided, however, that any references in these Bylaws to the 1934 Act or the rules and regulations thereunder are not intended to and shall not limit the requirements applicable to proposals and/or nominations to be considered pursuant to Section 5(a)(iii) of these Bylaws.
(h) For purposes of Sections 5 and 6 of these Bylaws,
(i) “public announcement” shall mean disclosure in a press release reported by the Dow Jones News Service, Associated Press or comparable national news service or in a document publicly filed by the corporation with the Securities and Exchange Commission pursuant to Section 13, 14 or 15(d) of the 1934 Act; and
(ii) “affiliates” and “associates” shall have the meanings set forth in Rule 405 under the Securities Act of 1933, as amended (the “1933 Act”).
Section 6. Special Meetings.
(a) Special meetings of the stockholders of the corporation may be called, for any purpose as is a proper matter for stockholder action under Delaware law, by (i) the Chairman of the Board of Directors, (ii) the Chief Executive Officer, or (iii) the Board of Directors pursuant to a resolution adopted by a majority of the total number of authorized directors (whether or not there exist any vacancies in previously authorized directorships at the time any such resolution is presented to the Board of Directors for adoption).
(b) The Board of Directors shall determine the time and place, if any, of such special meeting. Upon determination of the time and place, if any, of the meeting, the Secretary shall cause a notice of meeting to be given to the stockholders entitled to vote, in accordance with the provisions of Section 7 of these Bylaws. No business may be transacted at such special meeting otherwise than specified in the notice of meeting.
(c) Nominations of persons for election to the Board of Directors may be made at a special meeting of stockholders at which directors are to be elected (i) by or at the direction of the Board of Directors or (ii) by any stockholder of the corporation who is a stockholder of record at the time of giving notice provided for in this paragraph, who shall be entitled to vote at the meeting and who delivers written notice to the Secretary of the corporation setting forth the information required by Section 5(b)(i) of these Bylaws. In the event the corporation calls a special meeting of stockholders for the purpose of electing one or more directors to the Board of Directors, any such stockholder of record may nominate a person or persons (as the case may be), for election to such position(s) as specified in the corporation’s notice of meeting, if written notice setting forth the information required by Section 5(b)(i) of these Bylaws shall be received by the Secretary at the principal executive offices of the corporation not later than the close of business on the later of the 90th day prior to such meeting or the 10th day following the day on which public
Annex L-4
announcement is first made of the date of the special meeting and of the nominees proposed by the Board of Directors to be elected at such meeting. The stockholder shall also update and supplement such information as required under Section 5(c) of these Bylaws. In no event shall an adjournment or a postponement of a special meeting for which notice has been given, or the public announcement thereof has been made, commence a new time period for the giving of a stockholder’s notice as described above.
(d) Notwithstanding the foregoing provisions of this Section 6, a stockholder must also comply with all applicable requirements of the 1934 Act and the rules and regulations thereunder with respect to matters set forth in this Section 6. Nothing in these Bylaws shall be deemed to affect any rights of stockholders to request inclusion of proposals in the corporation’s proxy statement pursuant to Rule 14a-8 under the 1934 Act; provided, however, that any references in these Bylaws to the 1934 Act or the rules and regulations thereunder are not intended to and shall not limit the requirements applicable to nominations for the election to the Board of Directors to be considered pursuant to Section 6(c) of these Bylaws.
Section 7. Notice of Meetings. Except as otherwise provided by law, notice, given in writing or by electronic transmission, of each meeting of stockholders shall be given not less than 10 nor more than 60 days before the date of the meeting to each stockholder entitled to vote at such meeting, such notice to specify the place, if any, date and hour, in the case of special meetings, the purpose or purposes of the meeting, and the means of remote communications, if any, by which stockholders and proxy holders may be deemed to be present in person and vote at any such meeting. If mailed, notice is deemed given when deposited in the United States mail, postage prepaid, directed to the stockholder at such stockholder’s address as it appears on the records of the corporation. If sent via electronic transmission, notice is deemed given as of the sending time recorded at the time of transmission. Notice of the time, place, if any, and purpose of any meeting of stockholders may be waived in writing, signed by the person entitled to notice thereof, or by electronic transmission by such person, either before or after such meeting, and will be waived by any stockholder by his attendance thereat in person, by remote communication, if applicable, or by proxy, except when the stockholder attends a meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Any stockholder so waiving notice of such meeting shall be bound by the proceedings of any such meeting in all respects as if due notice thereof had been given.
Section 8. Quorum. At all meetings of stockholders, except where otherwise provided by statute or by the corporation’s Amended and Restated Certificate of Incorporation (as amended or restated from time to time, the “Certificate of Incorporation”), or by these Bylaws, the presence, in person, by remote communication, if applicable, or by proxy duly authorized, of the holders of 33 1/3% of the outstanding shares of stock entitled to vote shall constitute a quorum for the transaction of business. In the absence of a quorum, any meeting of stockholders may be adjourned, from time to time, either by the chairman of the meeting or by vote of the holders of a majority of the shares represented thereat, but no other business shall be transacted at such meeting. The stockholders present at a duly called or convened meeting, at which a quorum is present, may continue to transact business until adjournment, notwithstanding the withdrawal of enough stockholders to leave less than a quorum. Except as otherwise provided by statute or by applicable stock exchange rules, or by the Certificate of Incorporation or these Bylaws, in all matters other than the election of directors, the affirmative vote of the majority of shares present in person, by remote communication, if applicable, or represented by proxy at the meeting and entitled to vote generally on the subject matter shall be the act of the stockholders. Except as otherwise provided by statute, the Certificate of Incorporation or these Bylaws, directors shall be elected by a plurality of the votes of the shares present in person, by remote communication, if applicable, or represented by proxy at the meeting and entitled to vote generally on the election of directors. Where a separate vote by a class or classes or series is required, except where otherwise provided by the statute or by the Certificate of Incorporation or these Bylaws, a majority of the outstanding shares of such class or classes or series, present in person, by remote communication, if applicable, or represented by proxy duly authorized, shall constitute a quorum entitled to take action with respect to that vote on that matter. Except where otherwise provided by statute or by the Certificate of Incorporation or these Bylaws, the affirmative vote of the majority (plurality, in the case of the election of directors) of shares of such class or classes or series present in person, by remote communication, if applicable, or represented by proxy at the meeting shall be the act of such class or classes or series.
Section 9. Adjournment and Notice of Adjourned Meetings. Any meeting of stockholders, whether annual or special, may be adjourned from time to time either by the chairman of the meeting or by the vote of a majority of the shares present in person, by remote communication, if applicable, or represented by proxy at the meeting. When a meeting is adjourned to another time or place, if any, notice need not be given of the adjourned meeting if the time and place, if any, thereof are announced at the meeting at which the adjournment is taken. At the adjourned meeting, the
Annex L-5
corporation may transact any business which might have been transacted at the original meeting. If the adjournment is for more than 30 days or if after the adjournment a new record date is fixed for the adjourned meeting, a notice of the adjourned meeting shall be given to each stockholder of record entitled to vote at the meeting.
Section 10. Voting Rights. For the purpose of determining those stockholders entitled to vote at any meeting of the stockholders, except as otherwise provided by law, only persons in whose names shares stand on the stock records of the corporation on the record date, as provided in Section 12 of these Bylaws, shall be entitled to vote at any meeting of stockholders. Every person entitled to vote shall have the right to do so either in person, by remote communication, if applicable, or by an agent or agents authorized by a proxy granted in accordance with Delaware law. An agent so appointed need not be a stockholder. No proxy shall be voted after three years from its date of creation unless the proxy provides for a longer period.
Section 11. Joint Owners of Stock. If shares or other securities having voting power stand of record in the names of two or more persons, whether fiduciaries, members of a partnership, joint tenants, tenants in common, tenants by the entirety, or otherwise, or if two or more persons have the same fiduciary relationship respecting the same shares, unless the Secretary is given written notice to the contrary and is furnished with a copy of the instrument or order appointing them or creating the relationship wherein it is so provided, their acts with respect to voting shall have the following effect: (a) if only one votes, his act binds all; (b) if more than one votes, the act of the majority so voting binds all; or (c) if more than one votes, but the vote is evenly split on any particular matter, each faction may vote the securities in question proportionally, or may apply to the Delaware Court of Chancery for relief as provided in the DGCL, Section 217(b). If the instrument filed with the Secretary shows that any such tenancy is held in unequal interests, a majority or even-split for the purpose of clause (c) of this Section 11 shall be a majority or even-split in interest.
Section 12. List of Stockholders. The Secretary shall prepare and make, at least 10 days before every meeting of stockholders, a complete list of the stockholders entitled to vote at said meeting, arranged in alphabetical order, showing the address of each stockholder and the number of shares registered in the name of each stockholder. Such list shall be open to the examination of any stockholder, for any purpose germane to the meeting, (a) on a reasonably accessible electronic network, provided that the information required to gain access to such list is provided with the notice of the meeting, or (b) during ordinary business hours, at the principal place of business of the corporation. In the event that the corporation determines to make the list available on an electronic network, the corporation may take reasonable steps to ensure that such information is available only to stockholders of the corporation.
Section 13. Action Without Meeting. No action shall be taken by the stockholders except at an annual or special meeting of stockholders called in accordance with these Bylaws, and no action shall be taken by the stockholders by written consent or electronic transmission.
Section 14. Organization.
(a) At every meeting of stockholders, the Chairman of the Board of Directors, or, if a Chairman has not been appointed or is absent, the President, or, if the President is absent, if applicable, the Lead Independent Director (as defined below), or, if the Lead Independent Director is absent, a chairman of the meeting chosen by a majority in interest of the stockholders entitled to vote, present in person or by proxy, shall act as chairman. The Secretary, or, in his or her absence, an Assistant Secretary directed to do so by the President, shall act as secretary of the meeting.
(b) The Board of Directors of the corporation shall be entitled to make such rules or regulations for the conduct of meetings of stockholders as it shall deem necessary, appropriate or convenient. Subject to such rules and regulations of the Board of Directors, if any, the chairman of the meeting shall have the right and authority to prescribe such rules, regulations and procedures and to do all such acts as, in the judgment of such chairman, are necessary, appropriate or convenient for the proper conduct of the meeting, including, without limitation, establishing an agenda or order of business for the meeting, rules and procedures for maintaining order at the meeting and the safety of those present, limitations on participation in such meeting to stockholders of record of the corporation and their duly authorized and constituted proxies and such other persons as the chairman shall permit, restrictions on entry to the meeting after the time fixed for the commencement thereof, limitations on the time allotted to questions or comments by participants and regulation of the opening and closing of the polls for balloting on matters which are to be voted on by ballot. The date and time of the opening and closing of the polls for each matter upon which the stockholders will vote at the meeting shall be announced at the meeting. Unless and to the extent determined by the Board of Directors or the chairman of the meeting, meetings of stockholders shall not be required to be held in accordance with rules of parliamentary procedure.
Annex L-6
ARTICLE IV
DIRECTORS
Section 15. Number and Term of Office. The Board of Directors shall consist of not less than one and not more than seven directors as fixed from time to time solely by resolution of a majority of the total number of directors that the corporation would have if there were no vacancies. Directors need not be stockholders unless so required by the Certificate of Incorporation. If for any cause, the directors shall not have been elected at an annual meeting, they may be elected as soon thereafter as convenient at a special meeting of the stockholders called for that purpose in the manner provided in these Bylaws. Each director shall serve until his or her successor is duly elected and qualified or until his or her earlier death, resignation or removal. No decrease in the number of directors constituting the Board of Directors shall shorten the term of any incumbent director.
Section 16. Powers. The business and affairs of the corporation shall be managed by or under the direction of the Board of Directors, except as may be otherwise provided by statute or by the Certificate of Incorporation.
Section 17. Vacancies. Unless otherwise provided in the Certificate of Incorporation, and subject to the rights of the holders of any series of Preferred Stock, any vacancies on the Board of Directors resulting from death, resignation, disqualification, removal or other causes and any newly created directorships resulting from any increase in the number of directors shall, unless the Board of Directors determines by resolution that any such vacancies or newly created directorships shall be filled by stockholders, be filled only by the affirmative vote of a majority of the directors then in office, even though less than a quorum of the Board of Directors, or by a sole remaining director, and not by the stockholders, provided, however, that whenever the holders of any class or classes of stock or series thereof are entitled to elect one or more directors by the provisions of the Certificate of Incorporation, vacancies and newly created directorships of such class or classes or series shall, unless the Board of Directors determines by resolution that any such vacancies or newly created directorships shall be filled by stockholders, be filled by a majority of the directors elected by such class or classes or series thereof then in office, or by a sole remaining director so elected, and not by the stockholders. Any director elected in accordance with the preceding sentence shall hold office for the remainder of the full term of the director for which the vacancy was created or occurred and until such director’s successor shall have been elected and qualified. A vacancy in the Board of Directors shall be deemed to exist under this Bylaw in the case of the death, removal or resignation of any director.
Section 18. Resignation. Any director may resign at any time by delivering his or her notice in writing or by electronic transmission to the Secretary, such resignation to specify whether it will be effective at a particular time. If no such specification is made, it shall be deemed effective at the time of delivery to the Secretary. When one or more directors shall resign from the Board of Directors, effective at a future date, a majority of the directors then in office, including those who have so resigned, shall have power to fill such vacancy or vacancies, the vote thereon to take effect when such resignation or resignations shall become effective, and each director so chosen shall hold office for the unexpired portion of the term of the director whose place shall be vacated and until his successor shall have been duly elected and qualified.
Section 19. Removal.
(a) Subject to the rights of any series of Preferred Stock to elect additional directors under specified circumstances, neither the Board of Directors nor any individual director may be removed without cause.
(b) Subject to any limitation imposed by law, any individual director or directors may be removed with cause by the affirmative vote of the holders of at least 66 2/3% of the voting power of all then outstanding shares of capital stock of the corporation entitled to vote generally at an election of directors.
Section 20. Meetings.
(a) Regular Meetings. Unless otherwise restricted by the Certificate of Incorporation, regular meetings of the Board of Directors may be held at any time or date and at any place within or without the State of Delaware which has been designated by the Board of Directors and publicized among all directors, either orally or in writing, by telephone, including a voice-messaging system or other system designed to record and communicate messages, facsimile, telegraph or telex, or by electronic mail or other electronic means. No further notice shall be required for regular meetings of the Board of Directors.
Annex L-7
(b) Special Meetings. Unless otherwise restricted by the Certificate of Incorporation, special meetings of the Board of Directors may be held at any time and place within or without the State of Delaware whenever called by the Chairman of the Board, the Chief Executive Officer or a majority of the authorized number of directors.
(c) Meetings by Electronic Communications Equipment. Any member of the Board of Directors, or of any committee thereof, may participate in a meeting by means of conference telephone or other communications equipment by means of which all persons participating in the meeting can hear each other, and participation in a meeting by such means shall constitute presence in person at such meeting.
(d) Notice of Special Meetings. Notice of the time and place of all special meetings of the Board of Directors shall be orally or in writing, by telephone, including a voice messaging system or other system or technology designed to record and communicate messages, facsimile, telegraph or telex, or by electronic mail or other electronic means, during normal business hours, at least 24 hours before the date and time of the meeting. If notice is sent by U.S. mail, it shall be sent by first class mail, charges prepaid, at least three days before the date of the meeting. Notice of any meeting may be waived in writing, or by electronic transmission, at any time before or after the meeting and will be waived by any director by attendance thereat, except when the director attends the meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened.
(e) Waiver of Notice. The transaction of all business at any meeting of the Board of Directors, or any committee thereof, however called or noticed, or wherever held, shall be as valid as though it had been transacted at a meeting duly held after regular call and notice, if a quorum be present and if, either before or after the meeting, each of the directors not present who did not receive notice shall sign a written waiver of notice or shall waive notice by electronic transmission. All such waivers shall be filed with the corporate records or made a part of the minutes of the meeting.
Section 21. Quorum and Voting.
(a) Unless the Certificate of Incorporation requires a greater number, and except with respect to questions related to indemnification arising under Section 44 of these Bylaws for which a quorum shall be one-third of the exact number of directors fixed from time to time, a quorum of the Board of Directors shall consist of a majority of the exact number of directors fixed from time to time by the Board of Directors in accordance with the Certificate of Incorporation; provided, however, at any meeting whether a quorum be present or otherwise, a majority of the directors present may adjourn from time to time until the time fixed for the next regular meeting of the Board of Directors, without notice other than by announcement at the meeting.
(b) At each meeting of the Board of Directors at which a quorum is present, all questions and business shall be determined by the affirmative vote of a majority of the directors present, unless a different vote be required by law, the Certificate of Incorporation or these Bylaws.
Section 22. Action Without Meeting. Unless otherwise restricted by the Certificate of Incorporation or these Bylaws, any action required or permitted to be taken at any meeting of the Board of Directors or of any committee thereof may be taken without a meeting, if all members of the Board of Directors or committee, as the case may be, consent thereto in writing or by electronic transmission, and such writing or writings or transmission or transmissions are filed with the minutes of proceedings of the Board of Directors or committee. Such filing shall be in paper form if the minutes are maintained in paper form and shall be in electronic form if the minutes are maintained in electronic form.
Section 23. Fees and Compensation. Directors shall be entitled to such compensation for their services as may be approved by the Board of Directors, including, if so approved, by resolution of the Board of Directors, a fixed sum and expenses of attendance, if any, for attendance at each regular or special meeting of the Board of Directors and at any meeting of a committee of the Board of Directors. Nothing herein contained shall be construed to preclude any director from serving the corporation in any other capacity as an officer, agent, employee, or otherwise and receiving compensation therefor.
Annex L-8
Section 24. Committees.
(a) Executive Committee. The Board of Directors may appoint an Executive Committee to consist of one or more members of the Board of Directors. The Executive Committee, to the extent permitted by law and provided in the resolution of the Board of Directors shall have and may exercise all the powers and authority of the Board of Directors in the management of the business and affairs of the corporation, and may authorize the seal of the corporation to be affixed to all papers which may require it; but no such committee shall have the power or authority in reference to (i) approving or adopting, or recommending to the stockholders, any action or matter (other than the election or removal of directors) expressly required by the DGCL to be submitted to stockholders for approval, or (ii) adopting, amending or repealing any Bylaw of the corporation.
(b) Other Committees. The Board of Directors may, from time to time, appoint such other committees as may be permitted by law. Such other committees appointed by the Board of Directors shall consist of one or more members of the Board of Directors and shall have such powers and perform such duties as may be prescribed by the resolution or resolutions creating such committees, but in no event shall any such committee have the powers denied to the Executive Committee in these Bylaws.
(c) Term. The Board of Directors, subject to any requirements of any outstanding series of Preferred Stock and the provisions of subsections (a) or (b) of this Section 24, may at any time increase or decrease the number of members of a committee or terminate the existence of a committee. The membership of a committee member shall terminate on the date of his death or voluntary resignation from the committee or from the Board of Directors. The Board of Directors may at any time for any reason remove any individual committee member and the Board of Directors may fill any committee vacancy created by death, resignation, removal or increase in the number of members of the committee. The Board of Directors may designate one or more directors as alternate members of any committee, who may replace any absent or disqualified member at any meeting of the committee, and, in addition, in the absence or disqualification of any member of a committee, the member or members thereof present at any meeting and not disqualified from voting, whether or not he or they constitute a quorum, may unanimously appoint another member of the Board of Directors to act at the meeting in the place of any such absent or disqualified member.
(d) Meetings. Unless the Board of Directors shall otherwise provide, regular meetings of the Executive Committee or any other committee appointed pursuant to this Section 24 shall be held at such times and places as are determined by the Board of Directors, or by any such committee, and when notice thereof has been given to each member of such committee, no further notice of such regular meetings need be given thereafter. Special meetings of any such committee may be held at any place which has been determined from time to time by such committee, and may be called by any director who is a member of such committee, upon notice to the members of such committee of the time and place of such special meeting given in the manner provided for the giving of notice to members of the Board of Directors of the time and place of special meetings of the Board of Directors. Notice of any special meeting of any committee may be waived in writing or by electronic transmission at any time before or after the meeting and will be waived by any director by attendance thereat, except when the director attends such special meeting for the express purpose of objecting, at the beginning of the meeting, to the transaction of any business because the meeting is not lawfully called or convened. Unless otherwise provided by the Board of Directors in the resolutions authorizing the creation of the committee, a majority of the authorized number of members of any such committee shall constitute a quorum for the transaction of business, and the act of a majority of those present at any meeting at which a quorum is present shall be the act of such committee.
Section 25. Duties of Chairman of the Board of Directors. The Chairman of the Board of Directors, when present, shall preside at all meetings of the stockholders and the Board of Directors. The Chairman of the Board of Directors shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers, as the Board of Directors shall designate from time to time.
Section 26. Lead Independent Director. The Chairman of the Board of Directors, or if the Chairman is not an independent director, one of the independent directors, may be designated by the Board of Directors as lead independent director (“Lead Independent Director”) to serve until replaced by the Board of Directors. The Lead Independent Director will: with the Chairman of the Board of Directors, establish the agenda for regular Board meetings and serve as chairman of Board of Directors meetings in the absence of the Chairman of the Board of Directors; establish the agenda for meetings of the independent directors; coordinate with the committee chairs regarding meeting agendas and informational requirements; preside over meetings of the independent directors; preside over any portions of meetings of the Board of Directors at which the evaluation or compensation of the Chief Executive Officer is presented
Annex L-9
or discussed; preside over any portions of meetings of the Board of Directors at which the performance of the Board of Directors is presented or discussed; and perform such other duties as may be established or delegated by the Chairman of the Board of Directors.
Section 27. Organization. At every meeting of the directors, the Chairman of the Board of Directors, or, if a Chairman has not been appointed or is absent, the Lead Independent Director, or if the Lead Independent Director is absent, the Chief Executive Officer (if a director), or, if a Chief Executive Officer is absent, the President (if a director), or if the President is absent, the most senior Vice President (if a director), or, in the absence of any such person, a chairman of the meeting chosen by a majority of the directors present, shall preside over the meeting. The Secretary, or in his absence, any Assistant Secretary or other officer or director directed to do so by the Chairman, shall act as secretary of the meeting.
ARTICLE V
OFFICERS
Section 28. Officers Designated. The officers of the corporation shall include, if and when designated by the Board of Directors, the Chairman of the Board of Directors, the Chief Executive Officer, the President, one or more Vice Presidents, the Secretary, the Chief Financial Officer and the Treasurer. The Board of Directors may also appoint one or more Assistant Secretaries and Assistant Treasurers and such other officers and agents with such powers and duties as it shall deem necessary. The Board of Directors may assign such additional titles to one or more of the officers as it shall deem appropriate. Any one person may hold any number of offices of the corporation at any one time unless specifically prohibited therefrom by law. The salaries and other compensation of the officers of the corporation shall be fixed by or in the manner designated by the Board of Directors.
Section 29. Tenure and Duties of Officers.
(a) General. All officers shall hold office at the pleasure of the Board of Directors and until their successors shall have been duly elected and qualified, unless sooner removed. Any officer elected or appointed by the Board of Directors may be removed at any time by the Board of Directors. If the office of any officer becomes vacant for any reason, the vacancy may be filled by the Board of Directors.
(b) Duties of Chief Executive Officer. The Chief Executive Officer shall preside at all meetings of the stockholders and at all meetings of the Board of Directors, unless the Chairman of the Board of Directors or the Lead Independent Director has been appointed and is present. Unless an officer has been appointed Chief Executive Officer of the corporation, the President shall be the Chief Executive Officer of the corporation and shall, subject to the control of the Board of Directors, have general supervision, direction and control of the business and officers of the corporation. To the extent that a Chief Executive Officer has been appointed and no President has been appointed, all references in these Bylaws to the President shall be deemed references to the Chief Executive Officer. The Chief Executive Officer shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers, as the Board of Directors shall designate from time to time.
(c) Duties of President. The President shall preside at all meetings of the stockholders and at all meetings of the Board of Directors, unless the Chairman of the Board of Directors, the Lead Independent Director, or the Chief Executive Officer has been appointed and is present. Unless another officer has been appointed Chief Executive Officer of the corporation, the President shall be the Chief Executive Officer of the corporation and shall, subject to the control of the Board of Directors, have general supervision, direction and control of the business and officers of the corporation. The President shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers, as the Board of Directors shall designate from time to time.
(d) Duties of Vice Presidents. The Vice Presidents may assume and perform the duties of the President in the absence or disability of the President or whenever the office of President is vacant. The Vice Presidents shall perform other duties commonly incident to their office and shall also perform such other duties and have such other powers as the Board of Directors or the Chief Executive Officer, or, if the Chief Executive Officer has not been appointed or is absent, the President shall designate from time to time.
(e) Duties of Secretary. The Secretary shall attend all meetings of the stockholders and of the Board of Directors and shall record all acts and proceedings thereof in the minute book of the corporation. The Secretary shall give notice in conformity with these Bylaws of all meetings of the stockholders and of all meetings of the Board of
Annex L-10
Directors and any committee thereof requiring notice. The Secretary shall perform all other duties provided for in these Bylaws and other duties commonly incident to the office and shall also perform such other duties and have such other powers, as the Board of Directors shall designate from time to time. The President may direct any Assistant Secretary or other officer to assume and perform the duties of the Secretary in the absence or disability of the Secretary, and each Assistant Secretary shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time.
(f) Duties of Chief Financial Officer. The Chief Financial Officer shall keep or cause to be kept the books of account of the corporation in a thorough and proper manner and shall render statements of the financial affairs of the corporation in such form and as often as required by the Board of Directors or the President. The Chief Financial Officer, subject to the order of the Board of Directors, shall have the custody of all funds and securities of the corporation. The Chief Financial Officer shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time. To the extent that a Chief Financial Officer has been appointed and no Treasurer has been appointed, all references in these Bylaws to the Treasurer shall be deemed references to the Chief Financial Officer. The President may direct the Treasurer, if any, or any Assistant Treasurer, or the Controller or any Assistant Controller to assume and perform the duties of the Chief Financial Officer in the absence or disability of the Chief Financial Officer, and each Treasurer and Assistant Treasurer and each Controller and Assistant Controller shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time.
(g) Duties of Treasurer. Unless another officer has been appointed Chief Financial Officer of the corporation, the Treasurer shall be the chief financial officer of the corporation and shall keep or cause to be kept the books of account of the corporation in a thorough and proper manner and shall render statements of the financial affairs of the corporation in such form and as often as required by the Board of Directors or the President, and, subject to the order of the Board of Directors, shall have the custody of all funds and securities of the corporation. The Treasurer shall perform other duties commonly incident to the office and shall also perform such other duties and have such other powers as the Board of Directors or the President shall designate from time to time.
Section 30. Delegation of Authority. The Board of Directors may from time to time delegate the powers or duties of any officer to any other officer or agent, notwithstanding any provision hereof.
Section 31. Resignations. Any officer may resign at any time by giving notice in writing or by electronic transmission to the Board of Directors or to the President or to the Secretary. Any such resignation shall be effective when received by the person or persons to whom such notice is given, unless a later time is specified therein, in which event the resignation shall become effective at such later time. Unless otherwise specified in such notice, the acceptance of any such resignation shall not be necessary to make it effective. Any resignation shall be without prejudice to the rights, if any, of the corporation under any contract with the resigning officer.
Section 32. Removal. Any officer may be removed from office at any time, either with or without cause, by the affirmative vote of a majority of the directors in office at the time, or by the unanimous written consent of the directors in office at the time, or by any committee or by the Chief Executive Officer or by other superior officers upon whom such power of removal may have been conferred by the Board of Directors.
ARTICLE VI
EXECUTION OF CORPORATE INSTRUMENTS AND VOTING OF SECURITIES OWNED BY THE CORPORATION
Section 33. Execution of Corporate Instruments. The Board of Directors may, in its discretion, determine the method and designate the signatory officer or officers, or other person or persons, to execute on behalf of the corporation any corporate instrument or document, or to sign on behalf of the corporation the corporate name without limitation, or to enter into contracts on behalf of the corporation, except where otherwise provided by law or these Bylaws, and such execution or signature shall be binding upon the corporation.
All checks and drafts drawn on banks or other depositaries on funds to the credit of the corporation or in special accounts of the corporation shall be signed by such person or persons as the Board of Directors shall authorize so to do.
Annex L-11
Unless authorized or ratified by the Board of Directors or within the agency power of an officer, no officer, agent or employee shall have any power or authority to bind the corporation by any contract or engagement or to pledge its credit or to render it liable for any purpose or for any amount.
Section 34. Voting of Securities Owned by the Corporation. All stock and other securities of other corporations owned or held by the corporation for itself, or for other parties in any capacity, shall be voted, and all proxies with respect thereto shall be executed, by the person authorized so to do by resolution of the Board of Directors, or, in the absence of such authorization, by the Chairman of the Board of Directors, the Chief Executive Officer, the President, or any Vice President.
ARTICLE VII
SHARES OF STOCK
Section 35. Form and Execution of Certificates. The shares of the corporation shall be represented by certificates, or shall be uncertificated if so provided by resolution or resolutions of the Board of Directors. Certificates for the shares of stock of the corporation, if any, shall be in such form as is consistent with the Certificate of Incorporation and applicable law. Every holder of stock represented by certificate in the corporation shall be entitled to have a certificate signed by or in the name of the corporation by the Chairman of the Board of Directors, the Chief Executive Officer, or the President or any Vice President and by the Chief Financial Officer, Treasurer or Assistant Treasurer or the Secretary or Assistant Secretary, certifying the number of shares owned by him in the corporation. Any or all of the signatures on the certificate may be facsimiles. In case any officer, transfer agent, or registrar who has signed or whose facsimile signature has been placed upon a certificate shall have ceased to be such officer, transfer agent, or registrar before such certificate is issued, it may be issued with the same effect as if he were such officer, transfer agent, or registrar at the date of issue.
Section 36. Lost Certificates. A new certificate or certificates shall be issued in place of any certificate or certificates theretofore issued by the corporation alleged to have been lost, stolen, or destroyed, upon the making of an affidavit of that fact by the person claiming the certificate of stock to be lost, stolen, or destroyed. The corporation may require, as a condition precedent to the issuance of a new certificate or certificates, the owner of such lost, stolen, or destroyed certificate or certificates, or the owner’s legal representative, to agree to indemnify the corporation in such manner as it shall require or to give the corporation a surety bond in such form and amount as it may direct as indemnity against any claim that may be made against the corporation with respect to the certificate alleged to have been lost, stolen, or destroyed.
Section 37. Transfers.
(a) Transfers of record of shares of stock of the corporation shall be made only upon its books by the holders thereof, in person or by attorney duly authorized, and, in the case of stock represented by certificate, upon the surrender of a properly endorsed certificate or certificates for a like number of shares.
(b) The corporation shall have power to enter into and perform any agreement with any number of stockholders of any one or more classes of stock of the corporation to restrict the transfer of shares of stock of the corporation of any one or more classes owned by such stockholders in any manner not prohibited by the DGCL.
Section 38. Fixing Record Dates.
(a) In order that the corporation may determine the stockholders entitled to notice of or to vote at any meeting of stockholders or any adjournment thereof, the Board of Directors may fix a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted by the Board of Directors, and which record date shall, subject to applicable law, not be more than 60 nor less than 10 days before the date of such meeting. If no record date is fixed by the Board of Directors, the record date for determining stockholders entitled to notice of or to vote at a meeting of stockholders shall be at the close of business on the day next preceding the day on which notice is given, or if notice is waived, at the close of business on the day next preceding the day on which the meeting is held. A determination of stockholders of record entitled to notice of or to vote at a meeting of stockholders shall apply to any adjournment of the meeting; provided, however, that the Board of Directors may fix a new record date for the adjourned meeting.
Annex L-12
(b) In order that the corporation may determine the stockholders entitled to receive payment of any dividend or other distribution or allotment of any rights or the stockholders entitled to exercise any rights in respect of any change, conversion or exchange of stock, or for the purpose of any other lawful action, the Board of Directors may fix, in advance, a record date, which record date shall not precede the date upon which the resolution fixing the record date is adopted, and which record date shall be not more than 60 days prior to such action. If no record date is fixed, the record date for determining stockholders for any such purpose shall be at the close of business on the day on which the Board of Directors adopts the resolution relating thereto.
Section 39. Registered Stockholders. The corporation shall be entitled to recognize the exclusive right of a person registered on its books as the owner of shares to receive dividends, and to vote as such owner, and shall not be bound to recognize any equitable or other claim to or interest in such share or shares on the part of any other person whether or not it shall have express or other notice thereof, except as otherwise provided by the laws of Delaware.
ARTICLE VIII
OTHER SECURITIES OF THE CORPORATION
Section 40. Execution of Other Securities. All bonds, debentures and other corporate securities of the corporation, other than stock certificates (covered in Section 35 of these Bylaws), may be signed by the Chairman of the Board of Directors, the Chief Executive Officer, the President or any Vice President, or such other person as may be authorized by the Board of Directors, and the corporate seal impressed thereon or a facsimile of such seal imprinted thereon and attested by the signature of the Secretary or an Assistant Secretary, or the Chief Financial Officer or Treasurer or an Assistant Treasurer; provided, however, that where any such bond, debenture or other corporate security shall be authenticated by the manual signature, or where permissible facsimile signature, of a trustee under an indenture pursuant to which such bond, debenture or other corporate security shall be issued, the signatures of the persons signing and attesting the corporate seal on such bond, debenture or other corporate security may be the imprinted facsimile of the signatures of such persons. Interest coupons appertaining to any such bond, debenture or other corporate security, authenticated by a trustee as aforesaid, shall be signed by the Treasurer or an Assistant Treasurer of the corporation or such other person as may be authorized by the Board of Directors, or bear imprinted thereon the facsimile signature of such person. In case any officer who shall have signed or attested any bond, debenture or other corporate security, or whose facsimile signature shall appear thereon or on any such interest coupon, shall have ceased to be such officer before the bond, debenture or other corporate security so signed or attested shall have been delivered, such bond, debenture or other corporate security nevertheless may be adopted by the corporation and issued and delivered as though the person who signed the same or whose facsimile signature shall have been used thereon had not ceased to be such officer of the corporation.
ARTICLE IX
DIVIDENDS
Section 41. Declaration of Dividends. Dividends upon the capital stock of the corporation, subject to the provisions of the Certificate of Incorporation and applicable law, if any, may be declared by the Board of Directors pursuant to law at any regular or special meeting. Dividends may be paid in cash, in property, or in shares of the capital stock, subject to the provisions of the Certificate of Incorporation and applicable law.
Section 42. Dividend Reserve. Before payment of any dividend, there may be set aside out of any funds of the corporation available for dividends such sum or sums as the Board of Directors from time to time, in their absolute discretion, think proper as a reserve or reserves to meet contingencies, or for equalizing dividends, or for repairing or maintaining any property of the corporation, or for such other purpose as the Board of Directors shall think conducive to the interests of the corporation, and the Board of Directors may modify or abolish any such reserve in the manner in which it was created.
ARTICLE X
FISCAL YEAR
Section 43. Fiscal Year. The fiscal year of the corporation shall be fixed by resolution of the Board of Directors.
Annex L-13
ARTICLE XI
INDEMNIFICATION
Section 44. Indemnification of Directors, Officers, Employees and Other Agents.
(a) Directors and Officers. The corporation shall indemnify its directors and officers to the extent not prohibited by the DGCL or any other applicable law; provided, however, that the corporation may modify the extent of such indemnification by individual contracts with its directors and officers; and, provided, further, that the corporation shall not be required to indemnify any director or officer in connection with any proceeding (or part thereof) initiated by such person unless (i) such indemnification is expressly required to be made by law, (ii) the proceeding was authorized by the Board of Directors of the corporation, (iii) such indemnification is provided by the corporation, in its sole discretion, pursuant to the powers vested in the corporation under the DGCL or any other applicable law or (iv) such indemnification is required to be made under subsection (d).
(b) Employees and Other Agents. The corporation shall have power to indemnify its employees and other agents as set forth in the DGCL or any other applicable law. The Board of Directors shall have the power to delegate the determination of whether indemnification shall be given to any such person (except for officers) or other persons as the Board of Directors shall determine.
(c) Expenses. The corporation shall advance to any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he is or was a director or officer, of the corporation, or is or was serving at the request of the corporation as a director or officer of another corporation, partnership, joint venture, trust or other enterprise, prior to the final disposition of the proceeding, promptly following request therefor, all expenses incurred by any director or officer in connection with such proceeding provided, however, that if the DGCL requires, an advancement of expenses incurred by a director or officer in his or her capacity as a director or officer (and not in any other capacity in which service was or is rendered by such indemnitee, including, without limitation, service to an employee benefit plan) shall be made only upon delivery to the corporation of an undertaking (hereinafter an “undertaking”), by or on behalf of such indemnitee, to repay all amounts so advanced if it shall ultimately be determined by final judicial decision from which there is no further right to appeal (hereinafter a “final adjudication”) that such indemnitee is not entitled to be indemnified for such expenses under this Section 44 or otherwise.
Notwithstanding the foregoing, unless otherwise determined pursuant to paragraph (e) of this Section 44, no advance shall be made by the corporation to an officer of the corporation (except by reason of the fact that such officer is or was a director of the corporation in which event this paragraph shall not apply) in any action, suit or proceeding, whether civil, criminal, administrative or investigative, if a determination is reasonably and promptly made (i) by a majority vote of directors who were not parties to the proceeding, even if not a quorum, or (ii) by a committee of such directors designated by a majority vote of such directors, even though less than a quorum, or (iii) if there are no such directors, or such directors so direct, by independent legal counsel in a written opinion, that the facts known to the decision-making party at the time such determination is made demonstrate clearly and convincingly that such person acted in bad faith or in a manner that such person did not believe to be in or not opposed to the best interests of the corporation.
(d) Enforcement. Without the necessity of entering into an express contract, all rights to indemnification and advances to directors and officers under this Section 44 shall be deemed to be contractual rights and be effective to the same extent and as if provided for in a contract between the corporation and the director or officer. Any right to indemnification or advances granted by this Section 44 to a director or officer shall be enforceable by or on behalf of the person holding such right in any court of competent jurisdiction if (i) the claim for indemnification or advances is denied, in whole or in part, or (ii) no disposition of such claim is made within 90 days of request therefor. To the extent permitted by law, the claimant in such enforcement action, if successful in whole or in part, shall be entitled to be paid also the expense of prosecuting the claim. In connection with any claim for indemnification, the corporation shall be entitled to raise as a defense to any such action that the claimant has not met the standards of conduct that make it permissible under the DGCL or any other applicable law for the corporation to indemnify the claimant for the amount claimed. In connection with any claim by an officer of the corporation (except in any action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that such officer is or was a director of the corporation) for advances, the corporation shall be entitled to raise a defense as to any such action clear and convincing evidence that such person acted in bad faith or in a manner that such person did not believe to be in
Annex L-14
or not opposed to the best interests of the corporation, or with respect to any criminal action or proceeding that such person acted without reasonable cause to believe that his conduct was lawful. Neither the failure of the corporation (including its Board of Directors, independent legal counsel or its stockholders) to have made a determination prior to the commencement of such action that indemnification of the claimant is proper in the circumstances because the director or officer has met the applicable standard of conduct set forth in the DGCL or any other applicable law, nor an actual determination by the corporation (including its Board of Directors, independent legal counsel or its stockholders) that the claimant has not met such applicable standard of conduct, shall be a defense to the action or create a presumption that claimant has not met the applicable standard of conduct. In any suit brought by a director or officer to enforce a right to indemnification or to an advancement of expenses hereunder, the burden of proving that the director or officer is not entitled to be indemnified, or to such advancement of expenses, under this Section 44 or otherwise shall be on the corporation.
(e) Non-Exclusivity of Rights. The rights conferred on any person by this Bylaw shall not be exclusive of any other right which such person may have or hereafter acquire under any applicable statute, provision of the Certificate of Incorporation, Bylaws, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in such person’s official capacity and as to action in another capacity while holding office. The corporation is specifically authorized to enter into individual contracts with any or all of its directors, officers, employees or agents respecting indemnification and advances, to the fullest extent not prohibited by the DGCL, or by any other applicable law.
(f) Survival of Rights. The rights conferred on any person by this Bylaw shall continue as to a person who has ceased to be a director or officer, or, if applicable, employee or other agent, and shall inure to the benefit of the heirs, executors and administrators of such a person.
(g) Insurance. To the fullest extent permitted by the DGCL or any other applicable law, the corporation, upon approval by the Board of Directors, may purchase insurance on behalf of any person required or permitted to be indemnified pursuant to this Section 44.
(h) Amendments. Any repeal or modification of this Section 44 shall only be prospective and shall not affect the rights under this Bylaw in effect at the time of the alleged occurrence of any action or omission to act that is the cause of any proceeding against any agent of the corporation.
(i) Saving Clause. If this Bylaw or any portion hereof shall be invalidated on any ground by any court of competent jurisdiction, then the corporation shall nevertheless indemnify each director and officer to the full extent not prohibited by any applicable portion of this Section 44 that shall not have been invalidated, or by any other applicable law. If this Section 44 shall be invalid due to the application of the indemnification provisions of another jurisdiction, then the corporation shall indemnify each director and officer to the full extent under any other applicable law.
(j) Certain Definitions. For the purposes of this Bylaw, the following definitions shall apply:
(i) The term “proceeding” shall be broadly construed and shall include, without limitation, the investigation, preparation, prosecution, defense, settlement, arbitration and appeal of, and the giving of testimony in, any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative.
(ii) The term “expenses” shall be broadly construed and shall include, without limitation, court costs, attorneys’ fees, witness fees, fines, amounts paid in settlement or judgment and any other costs and expenses of any nature or kind incurred in connection with any proceeding.
(iii) The term the “corporation” shall include, in addition to the resulting corporation, any constituent corporation (including any constituent of a constituent) absorbed in a consolidation or merger which, if its separate existence had continued, would have had power and authority to indemnify its directors, officers, and employees or agents, so that any person who is or was a director, officer, employee or agent of such constituent corporation, or is or was serving at the request of such constituent corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, shall stand in the same position under the provisions of this Section 44 with respect to the resulting or surviving corporation as he would have with respect to such constituent corporation if its separate existence had continued.
Annex L-15
(iv) References to a “director,” “officer,” “employee,” or “agent” of the corporation shall include, without limitation, situations where such person is serving at the request of the corporation as, respectively, a director, officer, employee, trustee or agent of another corporation, partnership, joint venture, trust or other enterprise.
(v) References to “other enterprises” shall include employee benefit plans; references to “fines” shall include any excise taxes assessed on a person with respect to an employee benefit plan; and references to “serving at the request of the corporation” shall include any service as a director, officer, employee or agent of the corporation which imposes duties on, or involves services by, such director, officer, employee, or agent with respect to an employee benefit plan, its participants, or beneficiaries; and a person who acted in good faith and in a manner such person reasonably believed to be in the interest of the participants and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interests of the corporation” as referred to in this Section 44.
ARTICLE XII
NOTICES
Section 46. Notices.
(a) Notice to Stockholders. Written notice to stockholders of stockholder meetings shall be given as provided in Section 7 of these Bylaws. Without limiting the manner by which notice may otherwise be given effectively to stockholders under any agreement or contract with such stockholder, and except as otherwise required by law, written notice to stockholders for purposes other than stockholder meetings may be sent by U.S. mail or nationally recognized overnight courier, or by facsimile, telegraph or telex, or by electronic mail or other electronic means.
(b) Notice to Directors. Any notice required to be given to any director may be given by the method stated in subsection (a), or as otherwise provided in these Bylaws, except that such notice other than one which is delivered personally shall be sent to such address as such director shall have filed in writing with the Secretary, or, in the absence of such filing, to the last known post office address of such director.
(c) Affidavit of Mailing. An affidavit of mailing, executed by a duly authorized and competent employee of the corporation or its transfer agent appointed with respect to the class of stock affected, or other agent, specifying the name and address or the names and addresses of the stockholder or stockholders, or director or directors, to whom any such notice or notices was or were given, and the time and method of giving the same, shall in the absence of fraud, be prima facie evidence of the facts therein contained.
(d) Methods of Notice. It shall not be necessary that the same method of giving notice be employed in respect of all recipients of notice, but one permissible method may be employed in respect of any one or more, and any other permissible method or methods may be employed in respect of any other or others.
(e) Notice to Person With Whom Communication is Unlawful. Whenever notice is required to be given, under any provision of law or of the Certificate of Incorporation or Bylaws of the corporation, to any person with whom communication is unlawful, the giving of such notice to such person shall not be required and there shall be no duty to apply to any governmental authority or agency for a license or permit to give such notice to such person. Any action or meeting which shall be taken or held without notice to any such person with whom communication is unlawful shall have the same force and effect as if such notice had been duly given. In the event that the action taken by the corporation is such as to require the filing of a certificate under any provision of the DGCL, the certificate shall state, if such is the fact and if notice is required, that notice was given to all persons entitled to receive notice except such persons with whom communication is unlawful.
(f) Notice to Stockholders Sharing an Address. Except as otherwise prohibited under the DGCL, any notice given under the provisions of the DGCL, the Certificate of Incorporation or the Bylaws shall be effective if given by a single written notice to stockholders who share an address if consented to by the stockholders at that address to whom such notice is given. Such consent shall have been deemed to have been given if such stockholder fails to object in writing to the corporation within 60 days of having been given notice by the corporation of its intention to send the single notice. Any consent shall be revocable by the stockholder by written notice to the corporation.
Annex L-16
ARTICLE XIII
AMENDMENTS
Section 47. Amendments. Subject to the limitations set forth in Section 44(h) of these Bylaws or the provisions of the Certificate of Incorporation, the Board of Directors is expressly empowered to adopt, amend or repeal the Bylaws of the corporation. Any adoption, amendment or repeal of the Bylaws of the corporation by the Board of Directors shall require the approval of a majority of the authorized number of directors. The stockholders also shall have power to adopt, amend or repeal the Bylaws of the corporation; provided, however, that, in addition to any vote of the holders of any class or series of stock of the corporation required by law or by the Certificate of Incorporation, such action by stockholders shall require the affirmative vote of the holders of at least 66 2/3% of the voting power of all of the then-outstanding shares of the capital stock of the corporation entitled to vote generally in the election of directors, voting together as a single class.
ARTICLE XIV
MISCELLANEOUS
Section 47. Forum.
(a) Unless the corporation consents in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if and only if the Court of Chancery of the State of Delaware lacks subject matter jurisdiction, any state court located within the State of Delaware or, if and only if all such state courts lack subject matter jurisdiction, the federal district court for the District of Delaware) and any appellate court therefrom shall be the sole and exclusive forum for the following claims or causes of action under Delaware statutory or common law: (A) any derivative claim or cause of action brought on behalf of the corporation; (B) any claim or cause of action for breach of a fiduciary duty owed by any current or former director, officer or other employee of the corporation, to the corporation or the corporation’s stockholders; (C) any claim or cause of action against the corporation or any current or former director, officer or other employee of the corporation, arising out of or pursuant to any provision of the DGCL, the Certificate of Incorporation or the Bylaws of the corporation (as each may be amended from time to time); (D) any claim or cause of action seeking to interpret, apply, enforce or determine the validity of the Certificate of Incorporation or the Bylaws of the corporation (as each may be amended from time to time, including any right, obligation, or remedy thereunder); (E) any claim or cause of action as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware; and (F) any claim or cause of action against the corporation or any current or former director, officer or other employee of the corporation, governed by the internal-affairs doctrine or otherwise related to the corporation’s internal affairs, in all cases to the fullest extent permitted by law and subject to the court having personal jurisdiction over the indispensable parties named as defendants. This Section 47 of Article XV shall not apply to claims or causes of action brought to enforce a duty or liability created by the 1933 Act or the 1934 Act or any other claim for which the federal courts have exclusive jurisdiction.
(b) Unless the corporation consents in writing to the selection of an alternative forum, to the fullest extent permitted by law, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the 1933 Act, including all causes of action asserted against any defendant named in such complaint. For the avoidance of doubt, this provision is intended to benefit and may be enforced by the corporation, its officers and directors, the underwriters for any offering giving rise to such complaint, and any other professional entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying the offering.
Adopted by the Board of Directors of the Corporation on April 11, 2025.
Annex L-17