FWP: Filing under Securities Act Rules 163/433 of free writing prospectuses
Published on February 10, 2025
Issuer
Free Writing Prospectus
Filed Pursuant to Rule 433
Registration Statement No. 333-282324
February 10, 2025

NYSE American (proposed) : APUS Issuer Free Writing Prospectus Filed Pursuant to Rule 433 Registration Statement No. 333 - 282324 February 10 , 2025

Forward - Looking Statements 2 NYSE AMERICAN (proposed): APUS This presentation contains "forward - looking statements" that are subject to substantial risks and uncertainties. All statements, other than statements of historical fact, contained in this presentation are forward - looking statements. Forward - looking statements contained in this presentation may be identified by the use of words such as "anticipate," "believe," "contemplate," "could," "estimate," "expect," "intend," "seek," "may," "might," "plan," "potential," "predict," "project," "target," "aim," "should," "will" "would," or the negative of these words or other similar expressions, although not all forward - looking statements contain these words. Forward - looking statements are based on Apimeds Pharmaceuticals US, Inc. current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Further, certain forward - looking statements are based on assumptions as to future events that may not prove to be accurate. These and other risks and uncertainties are described more fully in the sections titled "Risk Factors and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in the Company’s Registration Statement on Form S - 1 (No. 333 - 282324) and the amendments thereto with the Securities and Exchange Commission (the “SEC”). Forward - looking statements contained in this announcement are made as of this date, and Apimeds Pharmaceuticals US, Inc. undertakes no duty to update such information except as required under applicable law. Past performance is not indicative of future results. There is no guarantee that any specific outcome will be achieved. Investments may be speculative and there is a total loss of your investment. We have filed a registration statement (including a prospectus) on Form S - 1 (File No. 333 - 282324) with the SEC for the offering to which this communication relates. Before you invest, you should read the prospectus in that registration statement and other documents we have filed with the SEC for more complete information about Apimeds Pharmaceuticals US, Inc. and this offering. You may get these documents for free by visiting EDGAR on the SEC Web site at www.sec.gov . Alternatively, the issuer or any underwriter participating in the offering will arrange to send you the prospectus if you request it by contacting D. Boral Capital LLC, 590 Madison Ave 39th Floor, New York, NY 10022, by email at info@dboralcapital.com , or by telephone at +1 (212) 970 - 5150.
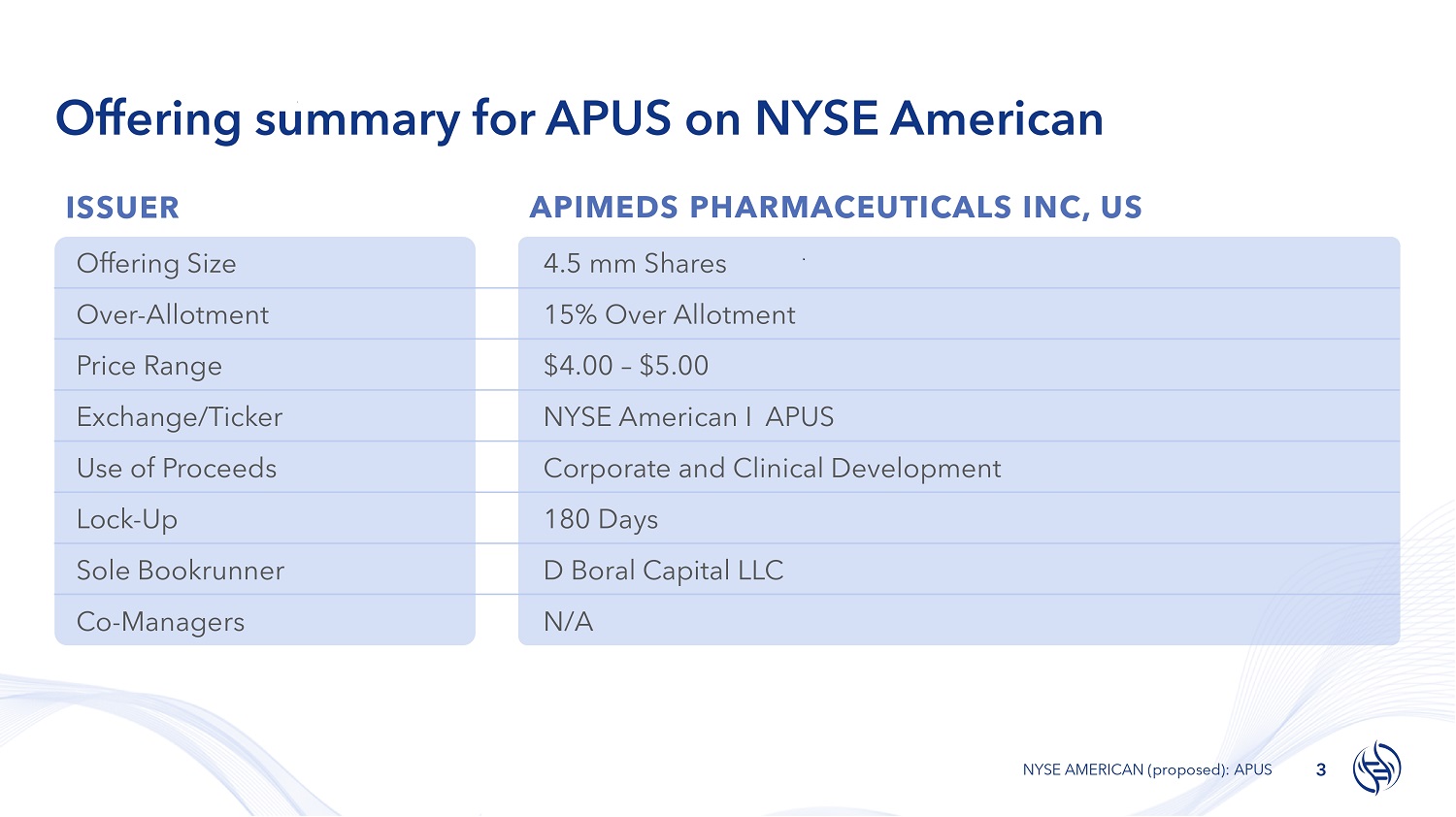
Offering summary for APUS on NYSE American ISSUER APIMEDS PHARMACEUTICALS INC, US 3 NYSE AMERICAN (proposed): APUS 4.5 mm Shares Offering Size 15% Over Allotment Over - Allotment $4.00 – $5.00 Price Range NYSE American I APUS Exchange/Ticker Corporate and Clinical Development Use of Proceeds 180 Days Lock - Up D Boral Capital LLC Sole Bookrunner N/A Co - Managers

Apimeds Pharmaceuticals US, Inc. Ready to Maximize the Apitox * Opportunity US company established in May 2020 • Based in Matawan, NJ • Subsidiary of Inscobee Inc • Form S - 1 filed with the SEC on September 25, 2024 Primary asset is Apitox, licensed from Apimeds Korea • Initially being developed as a potential treatment for pain and inflammation in patients with knee osteoarthritis Apitox has a demonstrated track record of real - world usage and experience, with facilities in South Korea administering Apitox commercially since 2003 • ~330 patients were treated in a previously conducted Phase 3 knee osteoarthritis trial, which demonstrated clinical efficacy and safety of Apitox in a US population • A follow - up FDA meeting in 2018 confirmed the need for a second confirmatory Phase 3 trial Clinical activity is projected to be completed in approximately 24 months, within the funding runway from the capital raise • Phase 3b study in osteoarthritis of the knee • Two corporate - sponsored investigator studies in inflammatory indications Strong management team and Board of Directors in place Erik Emerson, CEO Prior executive positions at Gilead, XOMA, Symplmed, Adhera, Mezzion Pharma, and Odyssey NeuroPharma Christopher Kim, MD, Chief Medical Officer A leading investigator in the field of bee therapy and co - author of the book Biotherapy: History, Principles and Practice, a practical guide to diagnosing and treating diseases using living organisms Mark Corrao, CFO A seasoned CFO with decades of experience serving as a CFO at public companies . His background includes over 40 years in accounting, finance, and public company regulations 4 *Tradename pending FDA review and approval NYSE AMERICAN (proposed): APUS
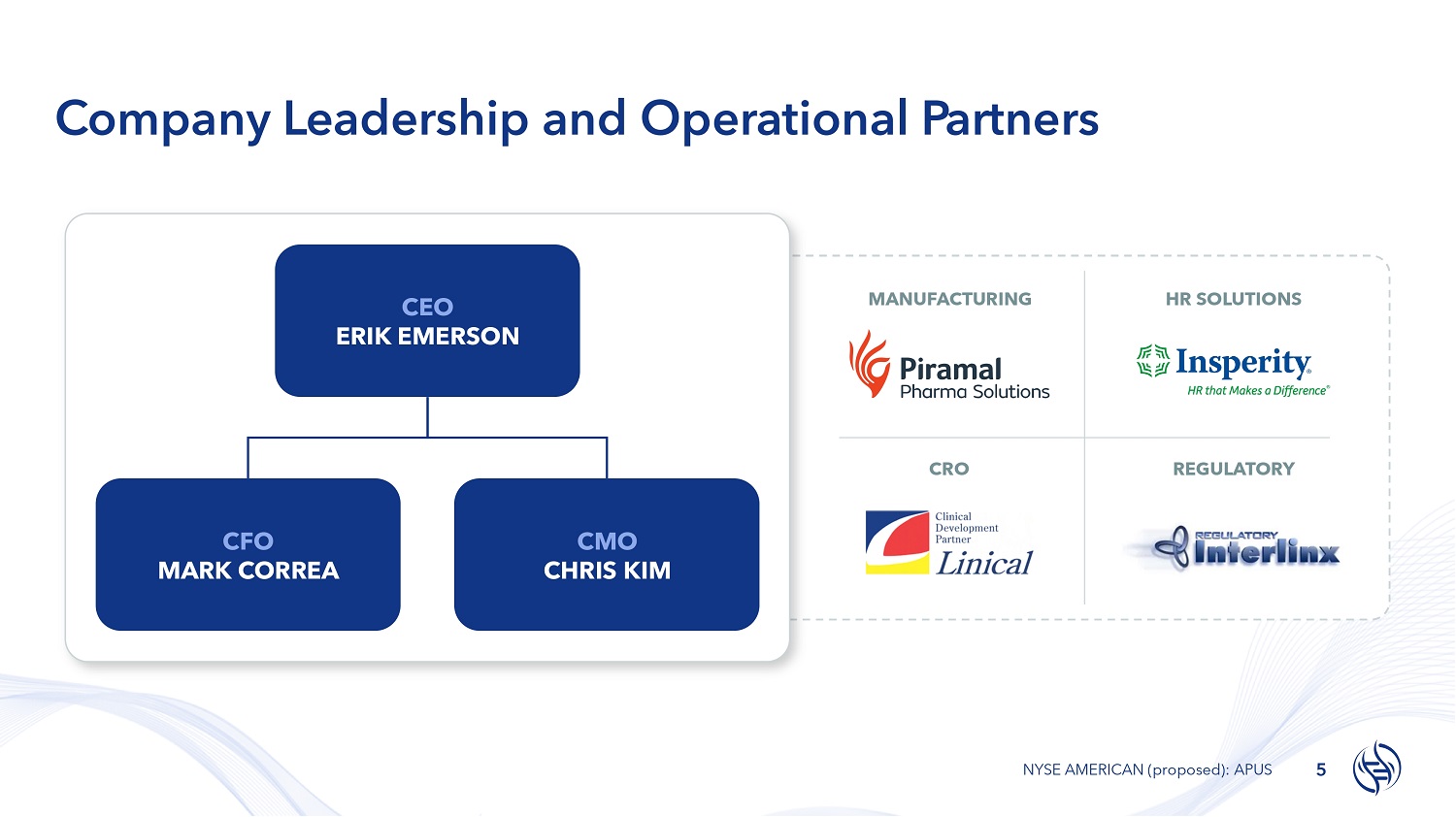
Company Leadership and Operational Partners MANUFACTURING HR SOLUTIONS CRO REGULATORY CFO MARK CORREA CMO CHRIS KIM CEO ERIK EMERSON 5 NYSE AMERICAN (proposed): APUS

Drive Immediate Executional Imperatives 1 Initiate a Phase 3 confirmatory trial in Knee Osteoarthritis 2 Launch a corporate - sponsored study in Multiple Sclerosis 3 Identify and pursue a 3rd development indication, initiating a corporate - sponsored study 6 NYSE AMERICAN (proposed): APUS

Apitox Positioned for Success in the US Market Differentiation from traditional therapies 1 3 7 NYSE AMERICAN (proposed): APUS • Apitox provides an alternative to NSAIDs, corticosteroids, and biologics, offering a new option for patients who have experienced limited efficacy or adverse effects • Positioning Apitox as an essential option for managing complex, chronic conditions 2 Potential efficacy across multiple indications • Honey - bee venom has demonstrated therapeutic potential in multiple inflammatory - related conditions, such as Knee OA and Multiple Sclerosis Untapped market potential • Apitox novel mechanism of action may fill gaps in the treatment of inflammation and immune modulation • With limited competition in this space , and extensive history of success for toxin related therapies, Apitox is well positioned for success

Differentiation from traditional therapies 1

Apitox Demonstrated efficacy with >20 years of safety A purified formulation of honeybee venom that has potent anti - inflammatory and analgesic properties A Phase 3 trial has demonstrated that patients experienced clinically significant pain relief and improved physical functioning Standard - of - care treatments such as NSAIDs and opioids come with risks of systemic toxicity, dependency, and abuse ; by contrast, Apitox offers a natural, non - addictive solution for managing joint pain Apitox may provide patients with a path to pain relief without the heavy burden of abuse potential, ushering in a new era of safer, non - addictive pain management Apitox is under consideration for its potential to modulate immune responses in other conditions, extending the lifecycle and value of Apitox 9 NYSE AMERICAN (proposed): APUS
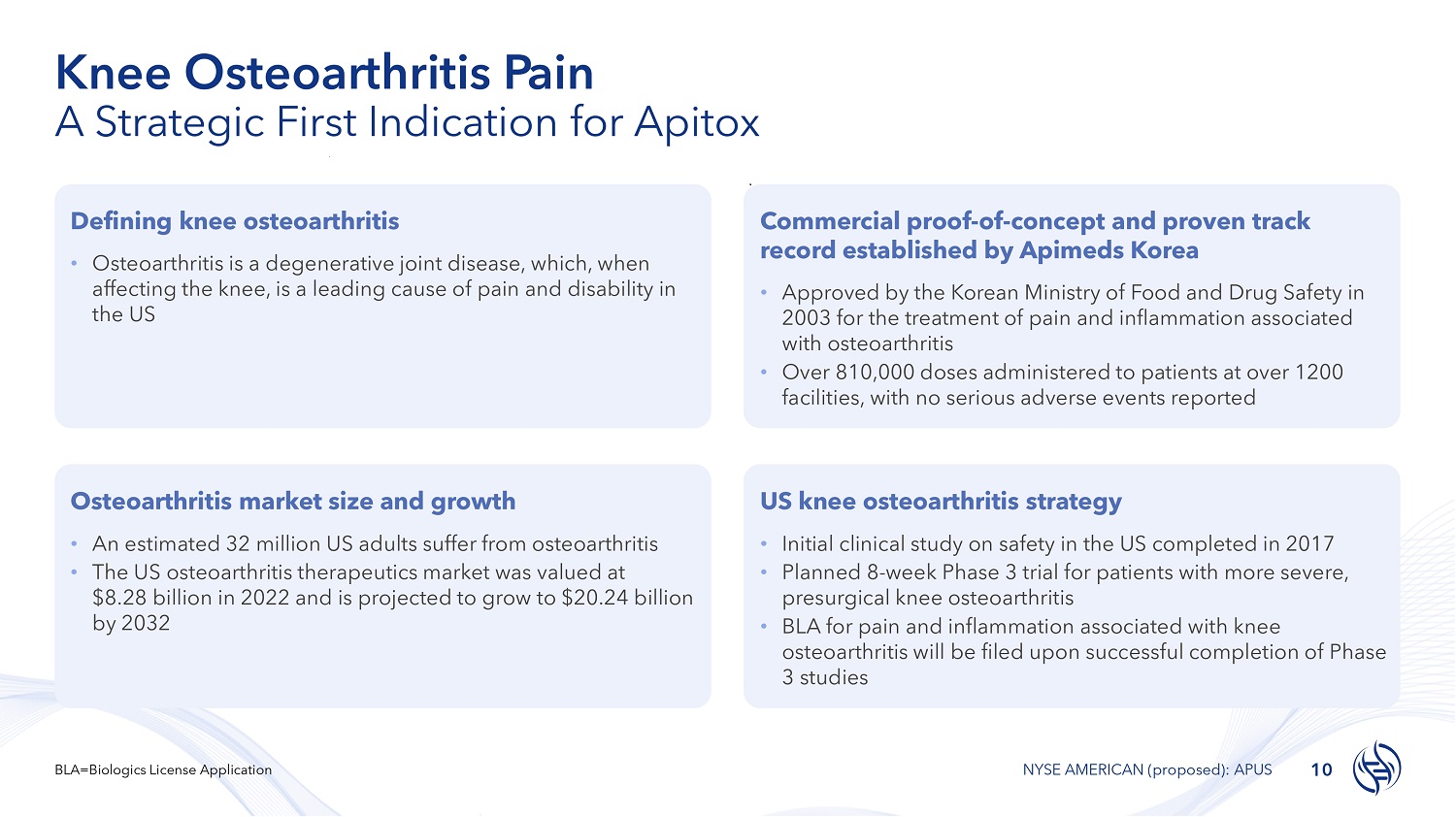
Knee Osteoarthritis Pain A Strategic First Indication for Apitox Defining knee osteoarthritis • Osteoarthritis is a degenerative joint disease, which, when affecting the knee, is a leading cause of pain and disability in the US Commercial proof - of - concept and proven track record established by Apimeds Korea • Approved by the Korean Ministry of Food and Drug Safety in 2003 for the treatment of pain and inflammation associated with osteoarthritis • Over 810,000 doses administered to patients at over 1200 facilities, with no serious adverse events reported Osteoarthritis market size and growth • An estimated 32 million US adults suffer from osteoarthritis • The US osteoarthritis therapeutics market was valued at $8.28 billion in 2022 and is projected to grow to $20.24 billion by 2032 US knee osteoarthritis strategy • Initial clinical study on safety in the US completed in 2017 • Planned 8 - week Phase 3 trial for patients with more severe, presurgical knee osteoarthritis • BLA for pain and inflammation associated with knee osteoarthritis will be filed upon successful completion of Phase 3 studies 10 BLA=Biologics License Application NYSE AMERICAN (proposed): APUS
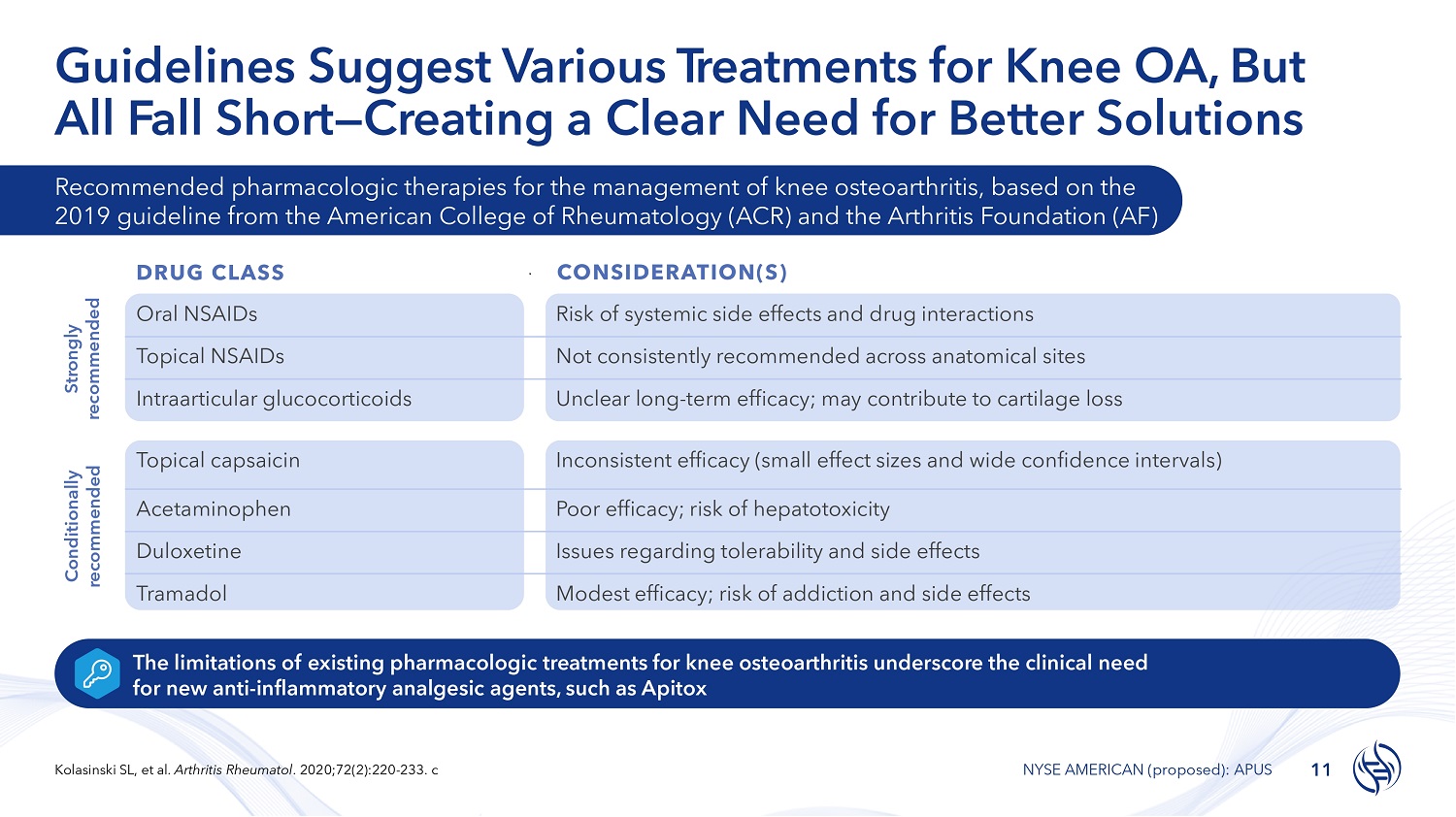
Guidelines Suggest Various T r e a t m e n ts for Knee OA, But All Fall Short — Creating a Clear Need for Better Solutions The limitations of existing pharmacologic treatments for knee osteoarthritis underscore the clinical need for new anti - inflammatory analgesic agents, such as Apitox Strongly recommended 11 Kolasinski SL, et al. Arthritis Rheumatol . 2020;72(2):220 - 233. c NYSE AMERICAN (proposed): APUS Conditionally recommended Recommended pharmacologic therapies for the management of knee osteoarthritis, based on the 2019 guideline from the American College of Rheumatology (ACR) and the Arthritis Foundation (AF) DRUG CLASS CONSIDERATION(S) Risk of systemic side effects and drug interactions Oral NSAIDs Not consistently recommended across anatomical sites Topical NSAIDs Unclear long - term efficacy; may contribute to cartilage loss Intraarticular glucocorticoids Inconsistent efficacy (small effect sizes and wide confidence intervals) Topical capsaicin Poor efficacy; risk of hepatotoxicity Acetaminophen Issues regarding tolerability and side effects Duloxetine Modest efficacy; risk of addiction and side effects Tramadol
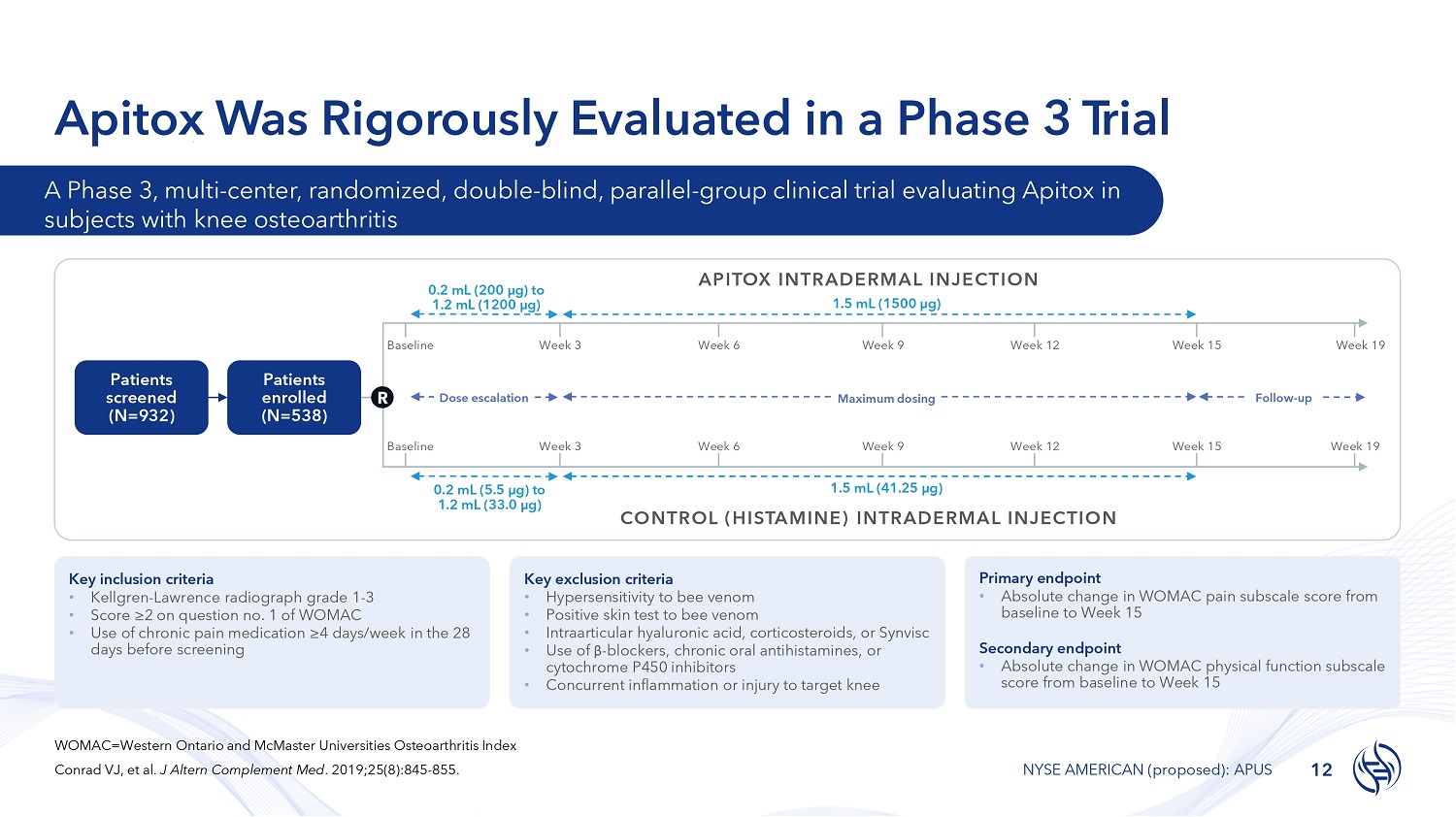
Apitox Was Rigorously Evaluated in a Phase 3 T rial Key inclusion criteria • Kellgren - Lawrence radiograph grade 1 - 3 • Score ≥2 on question no. 1 of WOMAC • Use of chronic pain medication ≥4 days/week in the 28 days before screening Key exclusion criteria • Hypersensitivity to bee venom • Positive skin test to bee venom • Intraarticular hyaluronic acid, corticosteroids, or Synvisc • Use of β - blockers, chronic oral antihistamines, or cytochrome P450 inhibitors • Concurrent inflammation or injury to target knee Primary endpoint • Absolute change in WOMAC pain subscale score from baseline to Week 15 Secondary endpoint • Absolute change in WOMAC physical function subscale score from baseline to Week 15 Patients screened (N= 932 ) Patients enrolled (N= 538 ) Week 3 Week 6 Week 9 Week 12 Week 15 Week 3 Week 15 Dose escalation Maximum dosing Baseline 0.2 mL (200 µg) to 1.2 mL (1200 µg) 0.2 mL (5.5 µg) to 1.2 mL (33.0 µg) APITOX INTRADERMAL INJECTION 1.5 mL (1500 µg) Week 6 Week 9 Week 12 1.5 mL (41.25 µg) CONTROL (HISTAMINE) INTRADERMAL INJECTION R Baseline Week 19 Week 19 Follow - up A Phase 3, multi - center, randomized, double - blind, parallel - group clinical trial evaluating Apitox in subjects with knee osteoarthritis WOMAC=Western Ontario and McMaster Universities Osteoarthritis Index Conrad VJ, et al. J Altern Complement Med . 2019;25(8):845 - 855. 12 NYSE AMERICAN (proposed): APUS
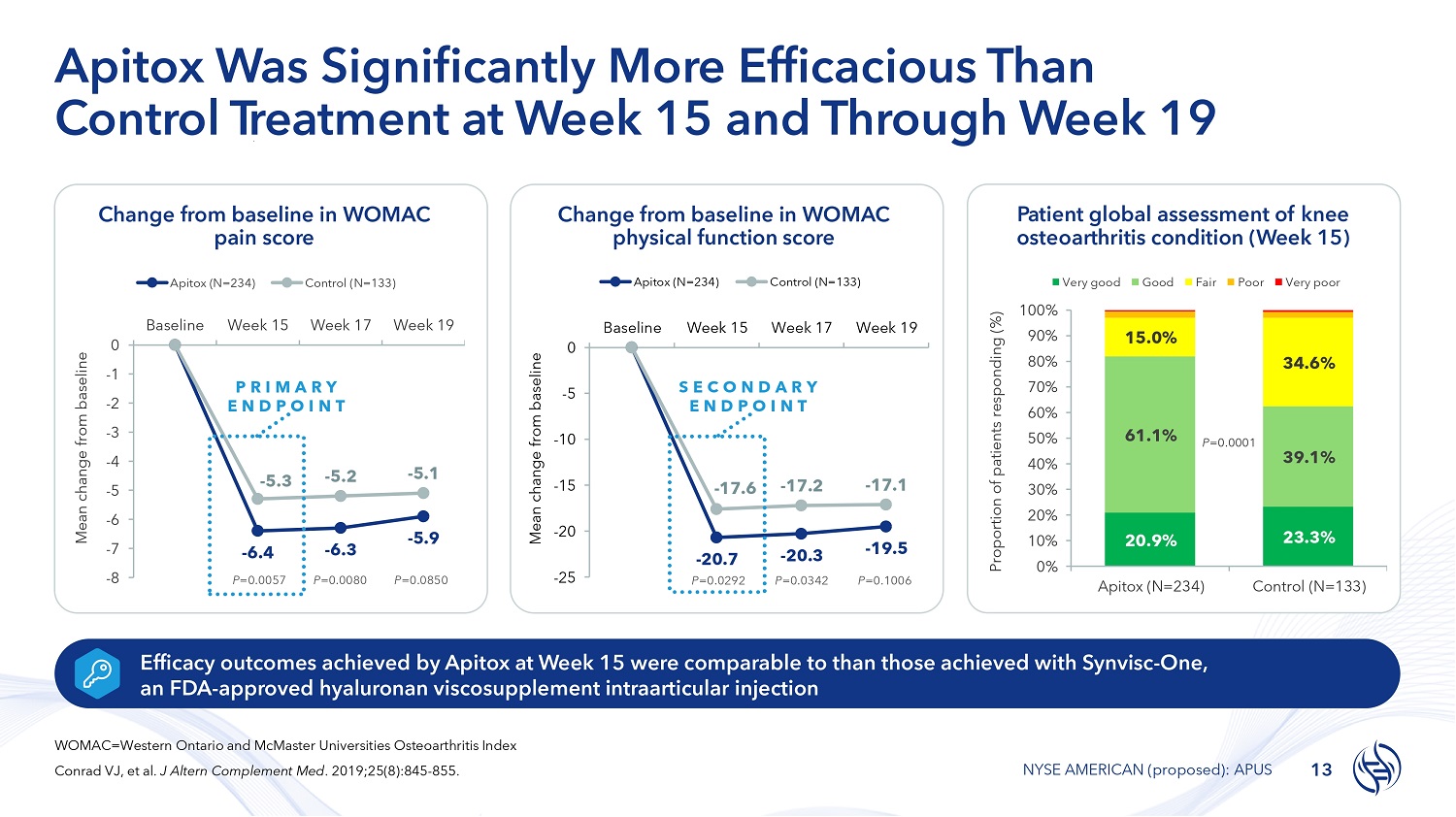
Change from baseline in WOMAC physical function score Patient global assessment of knee osteoarthritis condition (Week 15) Apitox Was Significantly More Efficacious Than Control T r e a t m e n t at Week 15 and Through Week 19 Efficacy outcomes achieved by Apitox at Week 15 were comparable to than those achieved with Synvisc - One, an FDA - approved hyaluronan viscosupplement intraarticular injection Change from baseline in WOMAC pain score - 5.9 - 5.3 - 5.2 - 5.1 0 - 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 Mean change from baseline Apitox (N=234) Baseline Week 15 Control (N=133) Week 17 Week 19 - 6.4 P =0.0057 - 6.3 P =0.0080 P =0.0850 P R I M A R Y E N D P O I N T - 17.6 - 17.2 - 17.1 - 25 - 20 - 15 - 10 - 5 0 Baseline Week 15 Week 17 Week 19 Mean change from baseline Apitox (N=234) Control (N=133) - 20.7 P =0.0292 - 20.3 P =0.0342 - 19.5 P =0.1006 S E C O N D A R Y E N D P O I N T 20.9% 23.3% 61.1% 39.1% 15.0% 34.6% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Apitox (N=234) Control (N=133) Proportion of patients responding (%) Very good Good Fair Poor Very poor P =0.0001 WOMAC=Western Ontario and McMaster Universities Osteoarthritis Index Conrad VJ, et al. J Altern Complement Med . 2019;25(8):845 - 855. 13 NYSE AMERICAN (proposed): APUS
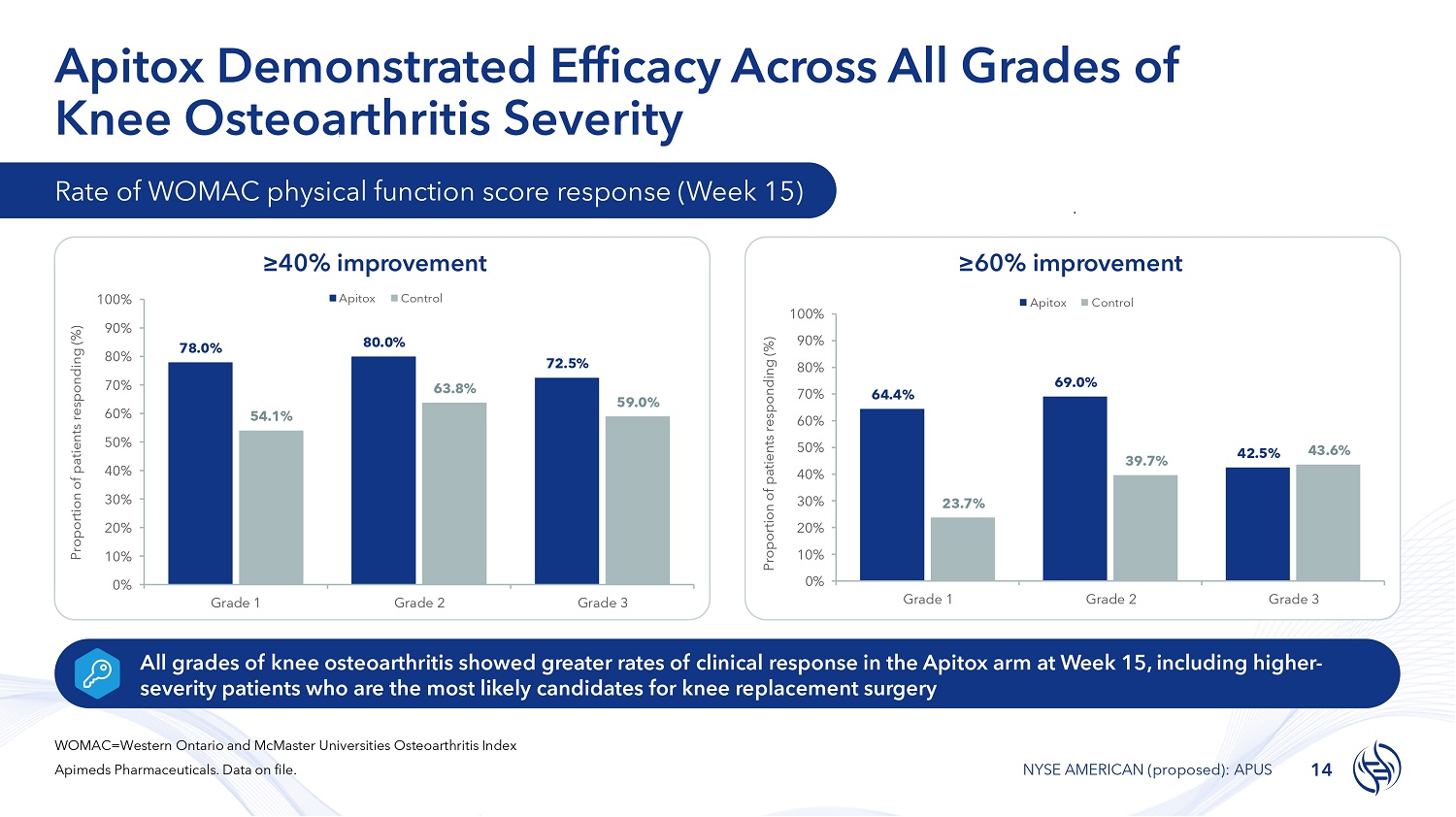
Apitox Demonstrated Efficacy Across All Grades of Knee Osteoarthritis Severity 78.0% 80.0% 72.5% 54.1% 63.8% 59.0% 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Grade 1 Grade 2 Grade 3 Proportion of patients responding (%) Apitox Control 64.4% 69.0% 42.5% 23.7% 39.7% 43.6% 70% 60% 50% 40% 30% 20% 10% 0% 100% 90% 80% Grade 1 Grade 2 Grade 3 Proportion of patients responding (%) Apitox Control Rate of WOMAC physical function score response (Week 15) ≥40% improvement ≥60% improvement All grades of knee osteoarthritis showed greater rates of clinical response in the Apitox arm at Week 15, including higher - severity patients who are the most likely candidates for knee replacement surgery WOMAC=Western Ontario and McMaster Universities Osteoarthritis Index Apimeds Pharmaceuticals. Data on file. 14 NYSE AMERICAN (proposed): APUS

FDA Feedback Confirmed Need for a Second Study to Support Initial Trial FDA meeting held on January 18, 2018 Notable comments regarding the additional trial • "Replicated evidence from two adequate and well - controlled studies will be required for your BLA." • "After extensive discussion, the Sponsor was told that they may submit the full results of Study 01 - 013 to the IND." • "The Division also recommended that the results from Study 01 - 013 be used to design future studies, and these studies should minimize dropouts and missing data." No safety issues raised • Will utilize post marketing surveillance from South Korea as a component of the safety database Review jurisdiction confirmed • Will be classified as a biologic with the Division of Anesthesia, Analgesia, and Addiction Products (DAAAP) 15 BLA=Biologics License Application; IND=Investigational New Drug (IND) application NYSE AMERICAN (proposed): APUS
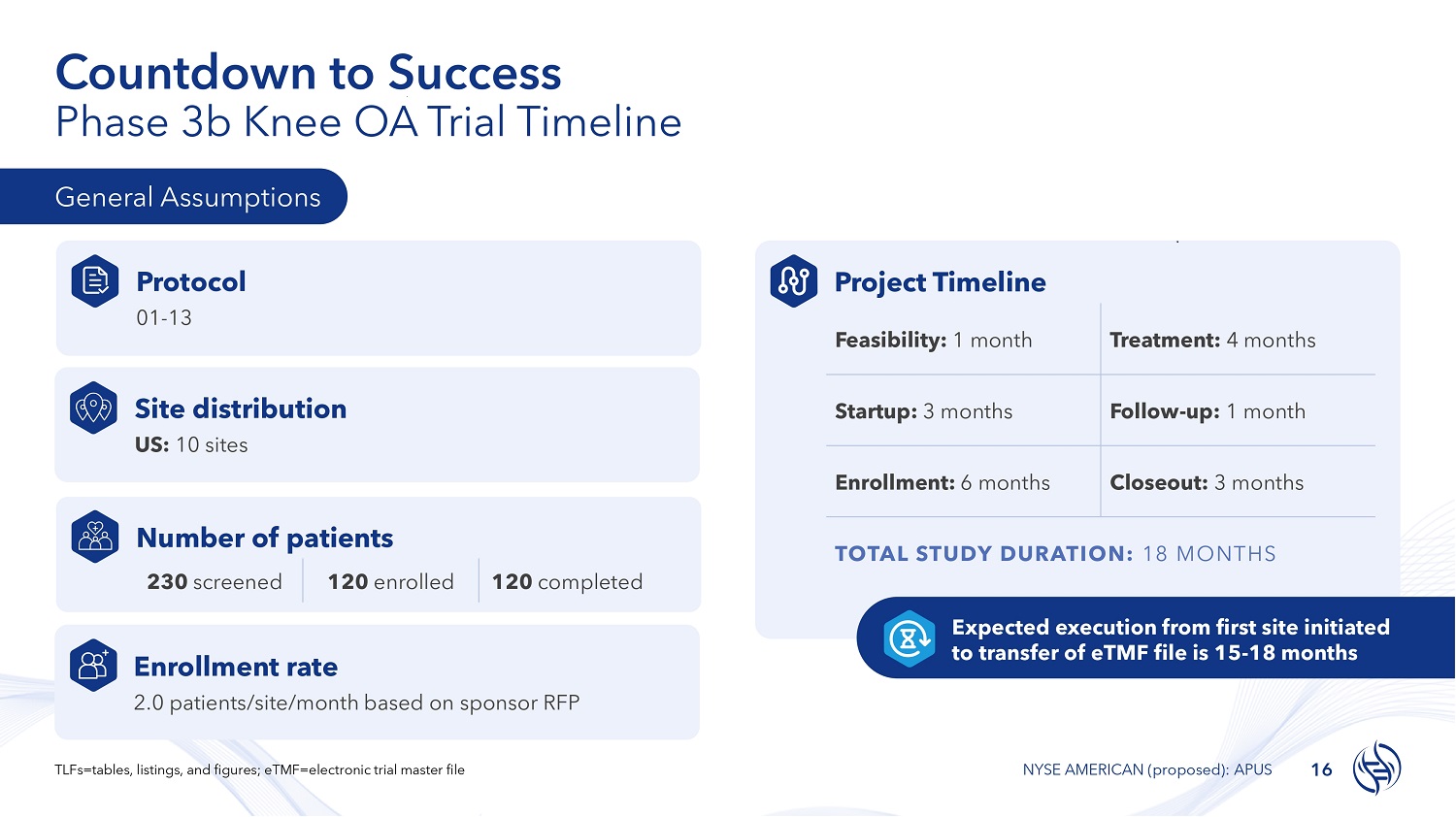
Countdown to Success Phase 3b Knee OA Trial Timeline General Assumptions Protocol 01 - 13 Site distribution US: 10 sites Number of patients 120 completed 120 enrolled 230 screened Project Timeline Treatment: 4 months Feasibility: 1 month Follow - up: 1 month Startup: 3 months Closeout: 3 months Enrollment: 6 months TOTAL STUDY DURATION: 18 MONTHS Enrollment rate 2.0 patients/site/month based on sponsor RFP Expected execution from first site initiated to transfer of eTMF file is 15 - 18 months 16 TLFs=tables, listings, and figures; eTMF=electronic trial master file NYSE AMERICAN (proposed): APUS

Potential efficacy across multiple indications 2

Unmet Need in Multiple Sclerosis (MS) Pain Management Presents a Key Opportunity for Apitox 18 MS is an autoimmune disease in which the immune system attacks the central nervous system (CNS) 1 • Primarily affects women between the ages of 20 and 50 2 • Causes immune - mediated damage to the myelin sheaths that protect neurons, leading to pain, fatigue, and other neurological symptoms 1 Disease - modifying agents, such as beta - interferons, have improved the outlook for MS patients, particularly those with relapsing/remitting MS (RRMS) 1 • However, most patients continue to experience symptoms 1 No drugs are currently approved for MS - related pain • Acorda’s Ampyra and its generics are the only supportive care drugs approved for MS - related walking difficulties 3,4 NYSE AMERICAN (proposed): APUS 1. McGinley M, et al. JAMA . 2021;325(8):765 - 779. 2. Milo R, Kahana E. Autoimmun Rev. 2010;9(5):A387 - A394. 3. Dunn J, Blight A. Curr Med Res Opin . 2011;27(7):1415 - 1423. 4. Ampyra prescribing information.
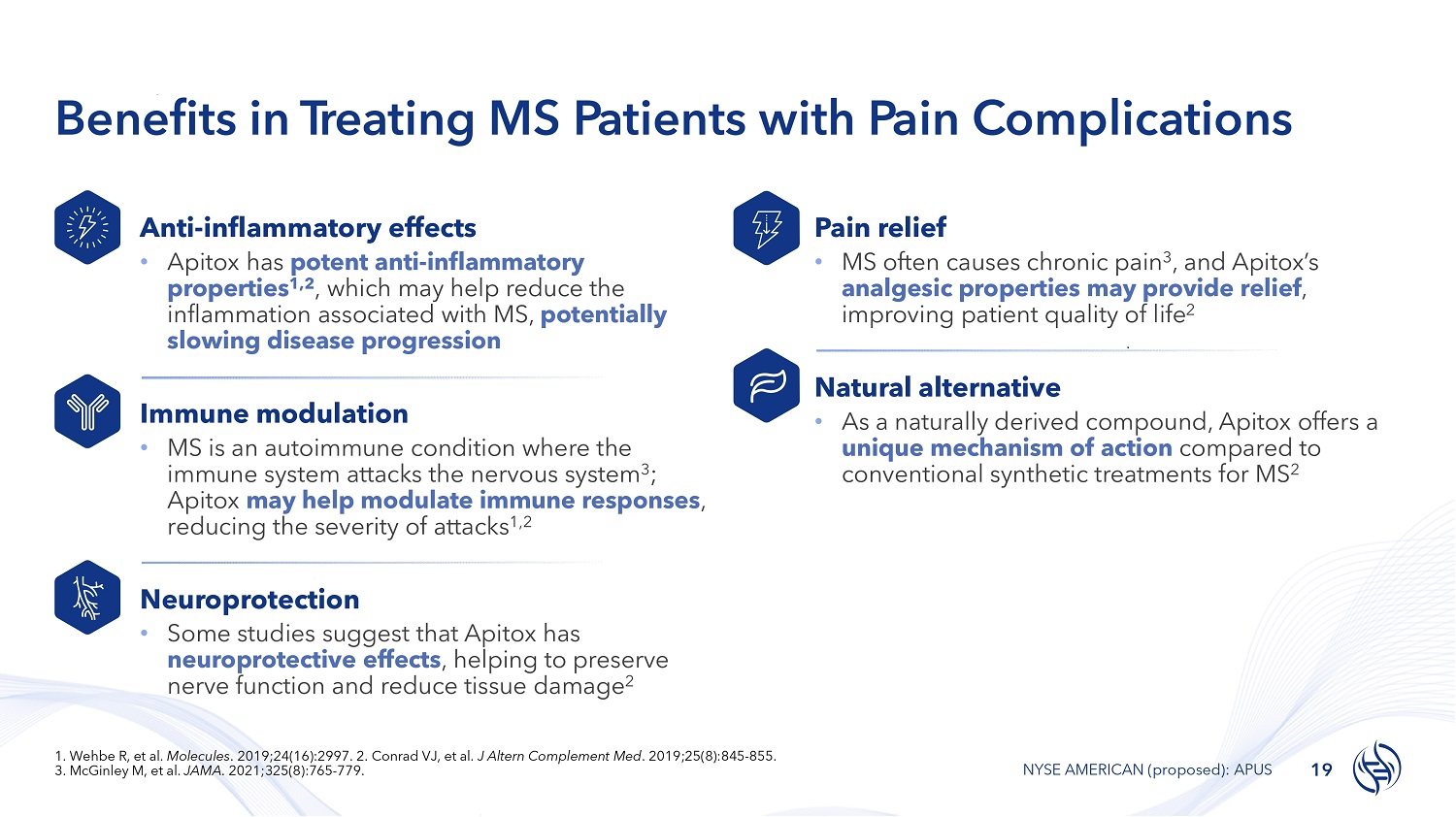
Benefits in T r e a t i n g MS Patients with Pain Complications Anti - inflammatory effects • Apitox has potent anti - inflammatory properties 1,2 , which may help reduce the inflammation associated with MS, potentially slowing disease progression Immune modulation • MS is an autoimmune condition where the immune system attacks the nervous system 3 ; Apitox may help modulate immune responses , reducing the severity of attacks 1,2 Neuroprotection • Some studies suggest that Apitox has neuroprotective effects , helping to preserve nerve function and reduce tissue damage 2 Pain relief • MS often causes chronic pain 3 , and Apitox’s analgesic properties may provide relief , improving patient quality of life 2 Natural alternative • As a naturally derived compound, Apitox offers a unique mechanism of action compared to conventional synthetic treatments for MS 2 19 NYSE AMERICAN (proposed): APUS 1. Wehbe R, et al. Molecules . 2019;24(16):2997. 2. Conrad VJ, et al. J Altern Complement Med . 2019;25(8):845 - 855. 3. McGinley M, et al. JAMA . 2021;325(8):765 - 779.
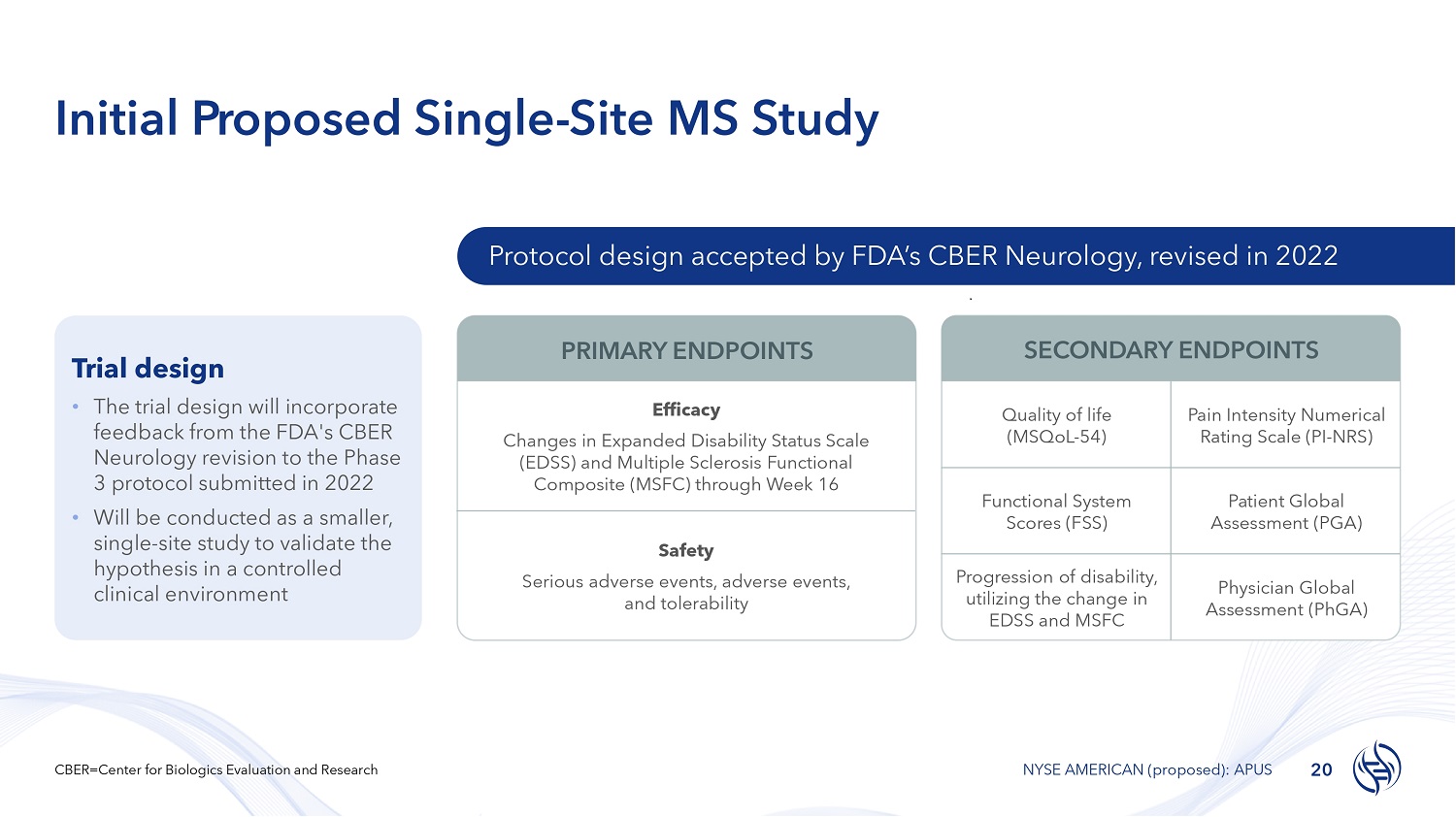
Initial Proposed Single - Site MS Study Trial design • The trial design will incorporate feedback from the FDA's CBER Neurology revision to the Phase 3 protocol submitted in 2022 • Will be conducted as a smaller, single - site study to validate the hypothesis in a controlled clinical environment Protocol design accepted by FDA’s CBER Neurology, revised in 2022 Efficacy Changes in Expanded Disability Status Scale (EDSS) and Multiple Sclerosis Functional Composite (MSFC) through Week 16 Safety Serious adverse events, adverse events, and tolerability Pain Intensity Numerical Rating Scale (PI - NRS) Quality of life (MSQoL - 54) Patient Global Assessment (PGA) Functional System Scores (FSS) Physician Global Assessment (PhGA) Progression of disability, utilizing the change in EDSS and MSFC PRIMARY ENDPOINTS SECONDARY ENDPOINTS 20 CBER=Center for Biologics Evaluation and Research NYSE AMERICAN (proposed): APUS
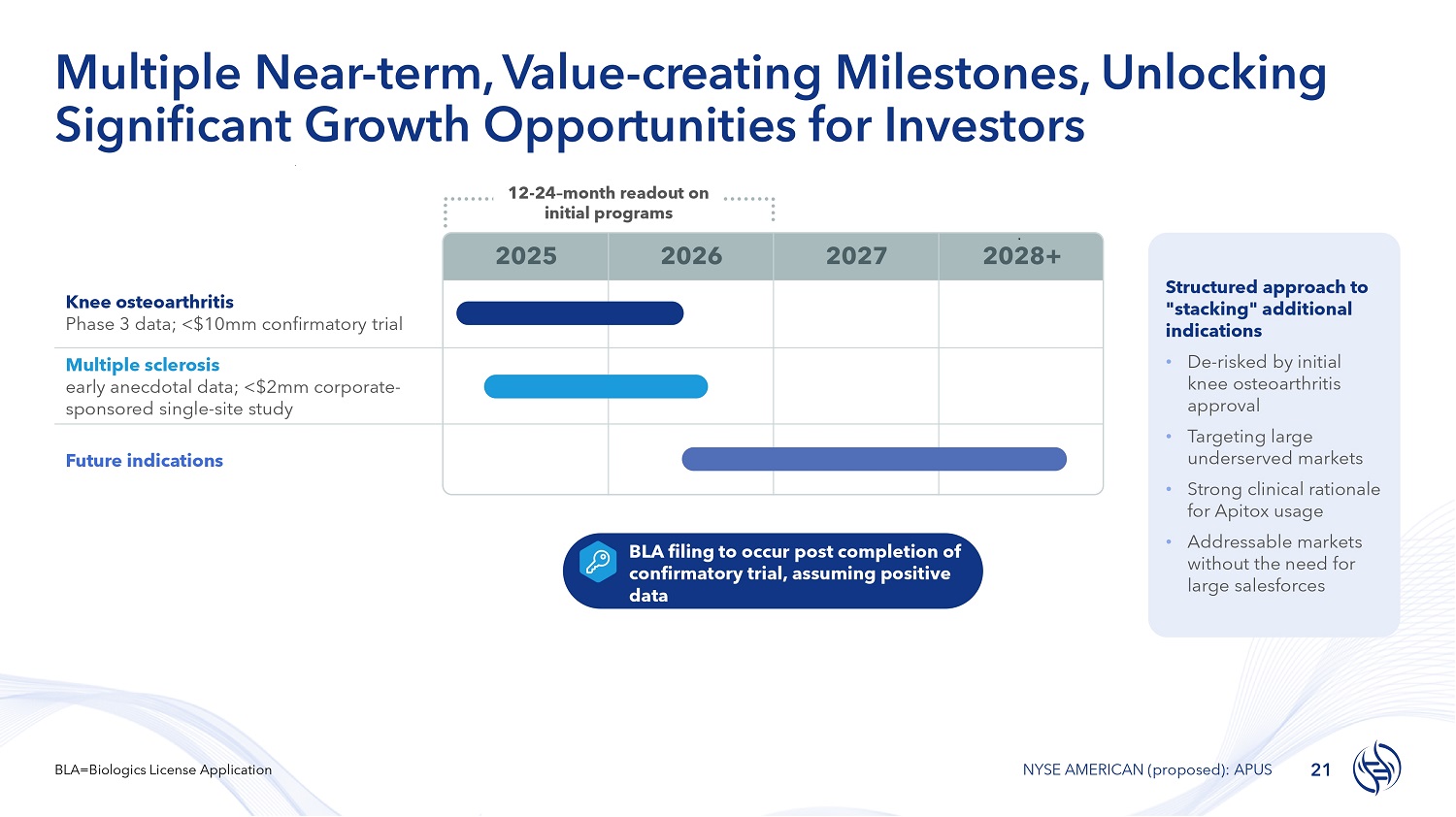
Multiple Near - term, Value - creating Milestones, Unlocking Significant Growth Opportunities for Investors Structured approach to "stacking" additional indications • De - risked by initial knee osteoarthritis approval • Targeting large underserved markets • Strong clinical rationale for Apitox usage • Addressable markets without the need for large salesforces 2028+ 2027 2026 2025 Knee osteoarthritis Phase 3 data; <$10mm confirmatory trial Multiple sclerosis early anecdotal data; <$2mm corporate - sponsored single - site study Future indications 12 - 24 – month readout on initial programs BLA filing to occur post completion of confirmatory trial, assuming positive data 21 BLA=Biologics License Application NYSE AMERICAN (proposed): APUS

Untapped market potential 3
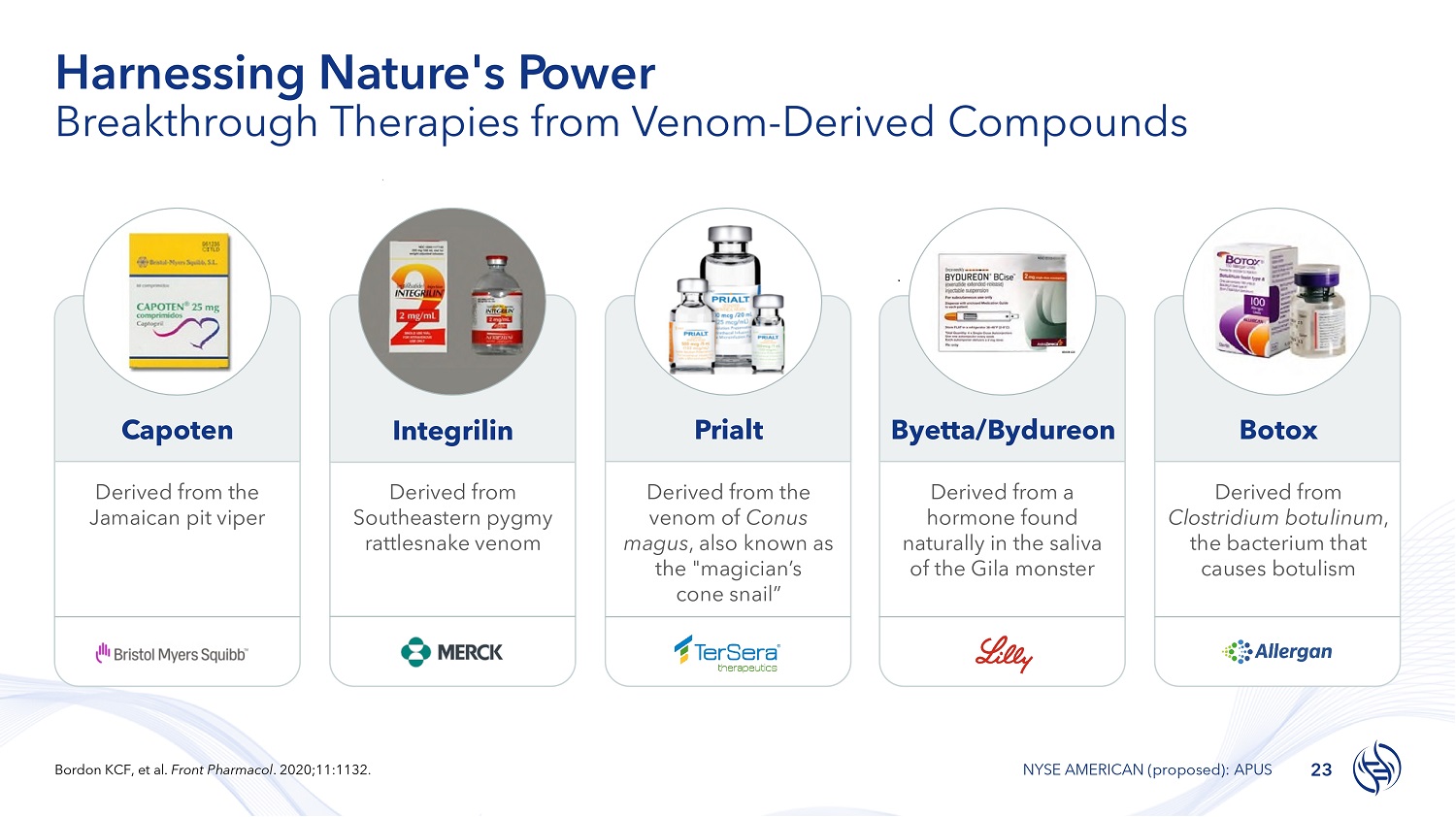
Harnessing Nature's Power Breakthrough Therapies from Venom - Derived Compounds Capoten Derived from the Jamaican pit viper Integrilin Derived from Southeastern pygmy rattlesnake venom Prialt Derived from the venom of Conus magus , also known as the "magician’s cone snail” Byetta/Bydureon Derived from a hormone found naturally in the saliva of the Gila monster Botox Derived from Clostridium botulinum , the bacterium that causes botulism 23 Bordon KCF, et al. Front Pharmacol . 2020;11:1132. NYSE AMERICAN (proposed): APUS
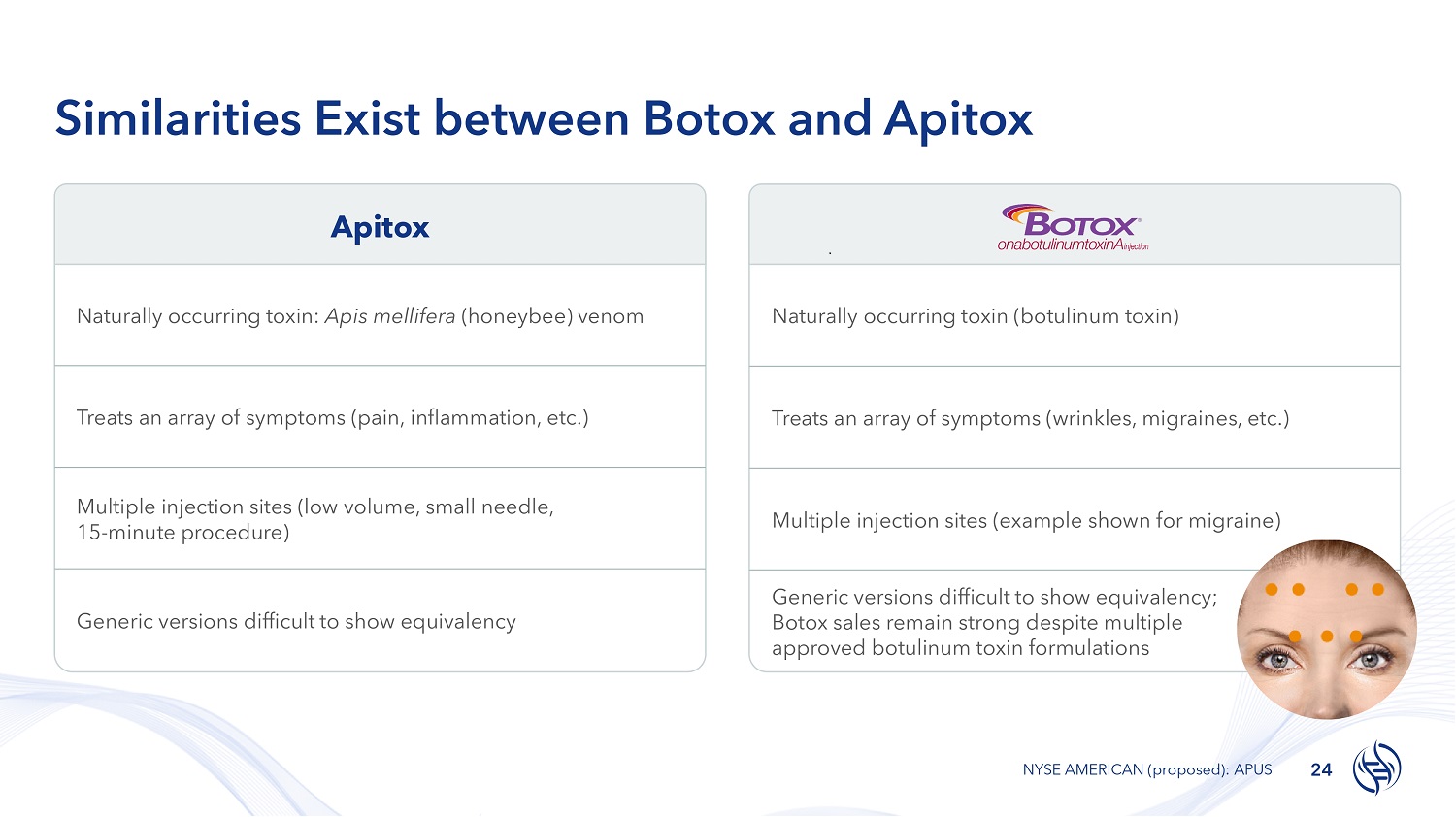
Apitox Similarities Exist between Botox and Apitox Naturally occurring toxin (botulinum toxin) Treats an array of symptoms (wrinkles, migraines, etc.) Multiple injection sites (example shown for migraine) Generic versions difficult to show equivalency; Botox sales remain strong despite multiple approved botulinum toxin formulations Naturally occurring toxin: Apis mellifera (honeybee) venom Treats an array of symptoms (pain, inflammation, etc.) Multiple injection sites (low volume, small needle, 15 - minute procedure) Generic versions difficult to show equivalency 24 NYSE AMERICAN (proposed): APUS
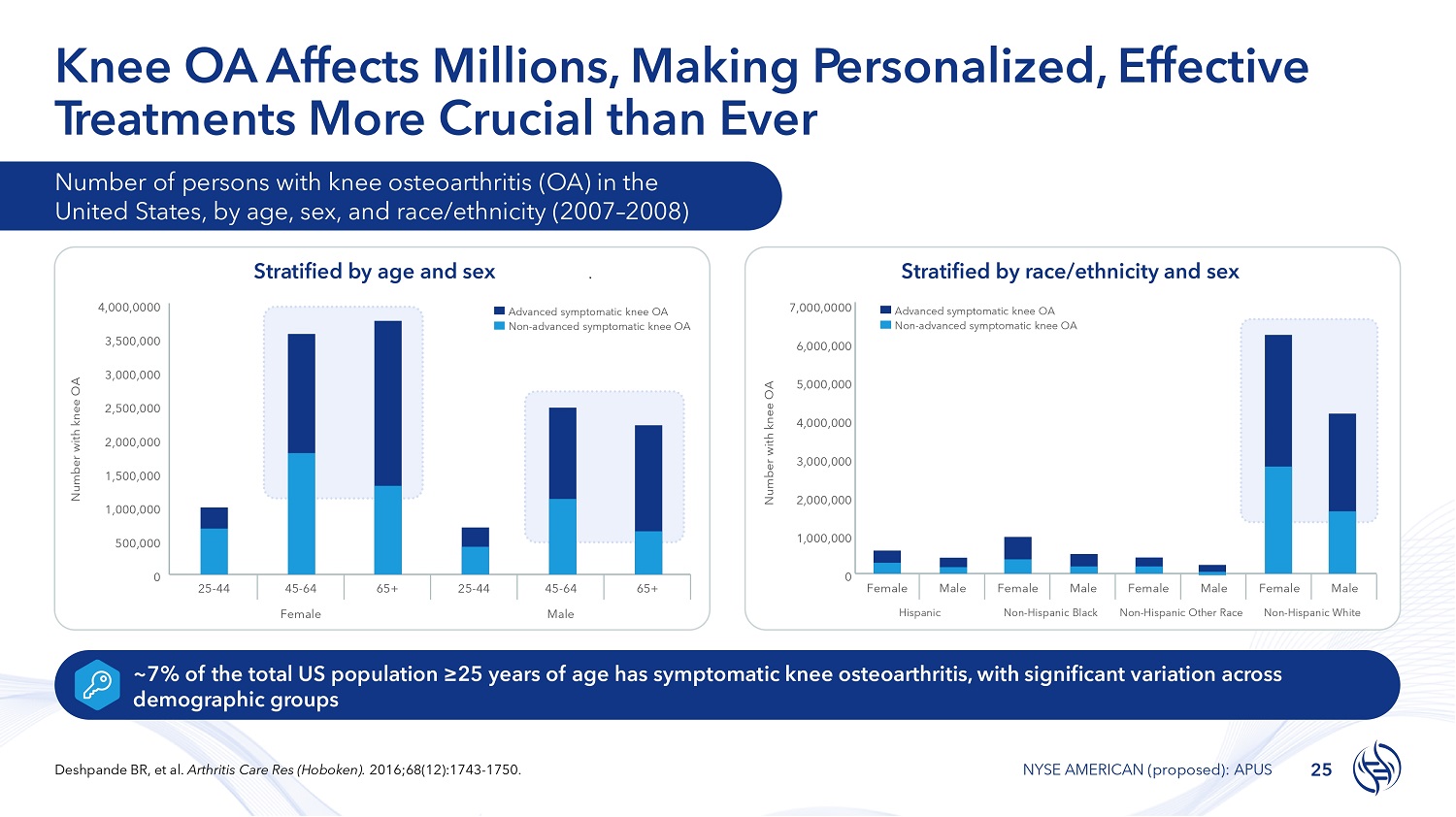
Knee OA Affects Millions, Making Personalized, Effective T r e a t m e n ts More Crucial than Ever Number of persons with knee osteoarthritis (OA) in the United States, by age, sex, and race/ethnicity (2007 – 2008) Stratified by age and sex ~7% of the total US population ≥25 years of age has symptomatic knee osteoarthritis, with significant variation across demographic groups 4,000,0000 3,500,000 3,000,000 2,500,000 2,000,000 1,500,000 1,000,000 500,000 0 Number with knee OA Advanced symptomatic knee OA Non - advanced symptomatic knee OA 7,000,0000 6,000,000 5,000,000 4,000,000 3,000,000 2,000,000 1,000,000 0 Number with knee OA Male Female Male Female Male Female Male Female Non - Hispanic White Non - Hispanic Other Race Non - Hispanic Black Hispanic Stratified by race/ethnicity and sex Advanced symptomatic knee OA Non - advanced symptomatic knee OA 65+ 45 - 64 25 - 44 65+ 45 - 64 25 - 44 Male Female 25 Deshpande BR, et al. Arthritis Care Res (Hoboken). 2016;68(12):1743 - 1750. NYSE AMERICAN (proposed): APUS
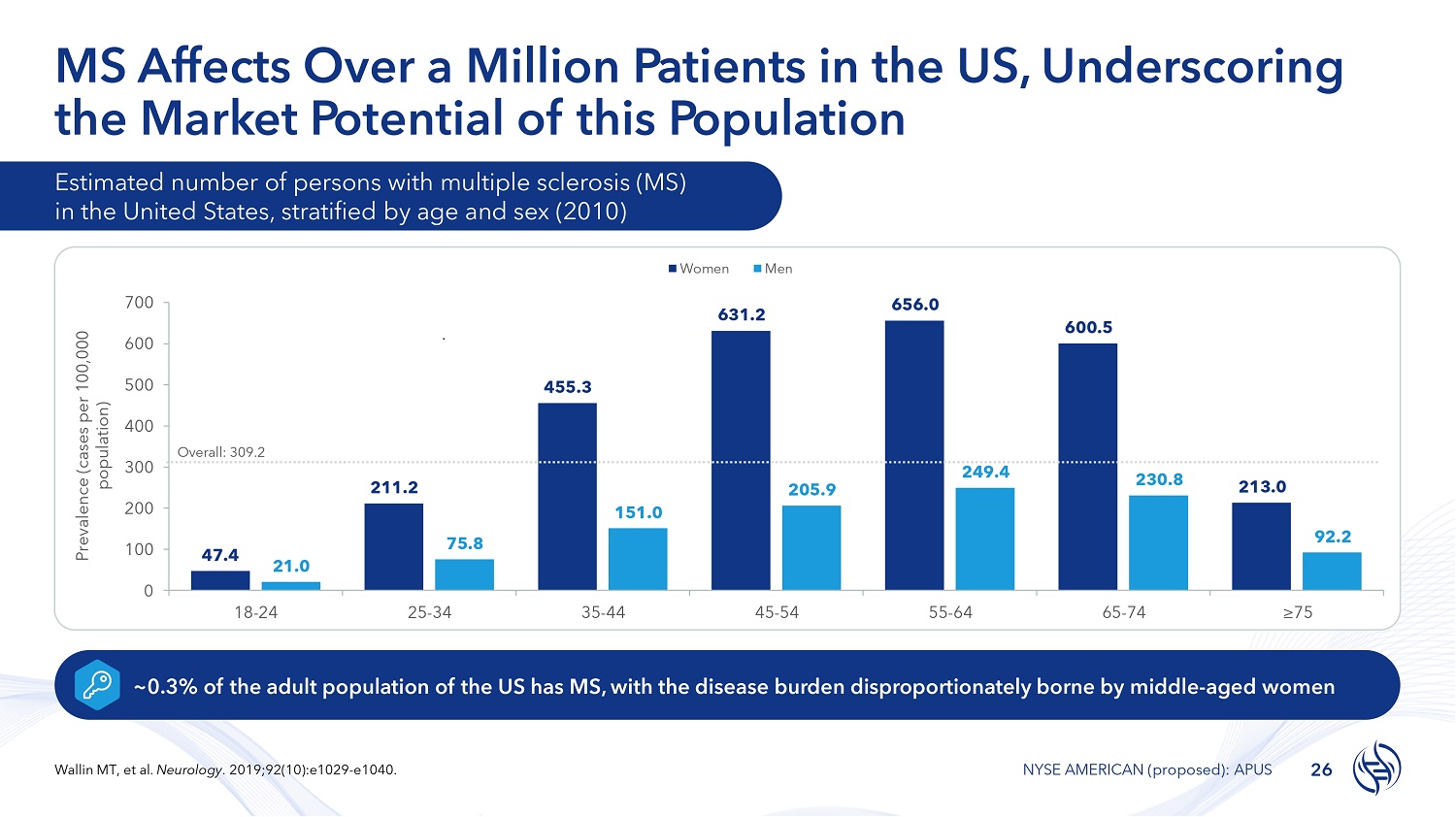
MS Affects Over a Million Patients in the US, Underscoring the Market Potential of this Population Estimated number of persons with multiple sclerosis (MS) in the United States, stratified by age and sex (2010) 47.4 211.2 455.3 631.2 656.0 600.5 213.0 21.0 75.8 151.0 205.9 249.4 230.8 92.2 0 100 200 300 500 600 700 18 - 24 25 - 34 35 - 44 45 - 54 55 - 64 65 - 74 ≥75 Prevalence (cases per 100,000 population) Women Men ~0.3% of the adult population of the US has MS, with the disease burden disproportionately borne by middle - aged women 400 Overall: 309.2 26 Wallin MT, et al. Neurology . 2019;92(10):e1029 - e1040. NYSE AMERICAN (proposed): APUS
Apitox's Novel Biologic Status Ensures 12 years of Market Exclusivity, Driving Long - term Growth Under Section 351(k)(7)(c), biologic entities newly approved through the Centers for Biologics Evaluation and Research (CBER) receive 12 years of market exclusivity from the date of approval • No products may seek approval using Apitox as a reference for 12 years post - approval We anticipate Apitox will operate independently of bee venom competition for the treatment of associated diseases for ≥12 years post - approval , if appropriate exclusivity granted 27 NYSE AMERICAN (proposed): APUS

Investing in the Company Behind Apitox A Proven Path to Market Leadership and Growth Untapped market with significant growth potential • Global market for inflammatory and autoimmune treatments is growing rapidly • Apitox offers a differentiated solution with significant potential to capture market share Proven efficacy with unique mechanism of action • Apitox’s unique mechanism of action, differentiates it from conventional therapies • Natural composition appeals to both patients and HCPs , offering a competitive edge Innovative product with expanding indications • Apitox has shown to be a potential therapeutic option for the treatment of Knee OA and Multiple Sclerosis • The broad therapeutic potential opens new revenue streams Experienced leadership and strategic vision • Company’s leadership team brings years of expertise in the biopharmaceutical industry , with a proven track record of bringing innovative therapies to market 28 NYSE AMERICAN (proposed): APUS
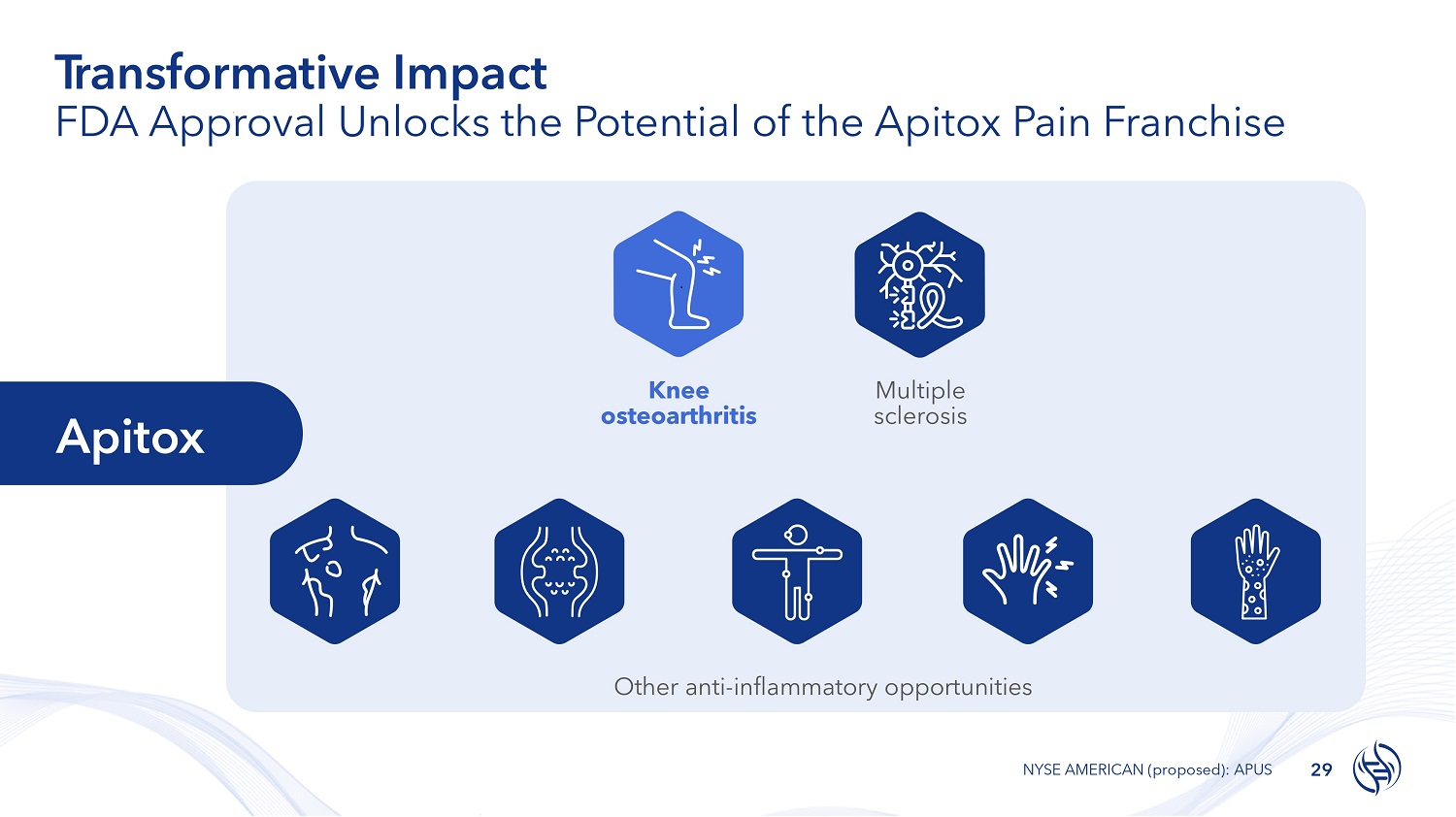
T r ans f or m a t i v e Impact FDA Approval Unlocks the Potential of the Apitox Pain Franchise 29 NYSE AMERICAN (proposed): APUS Knee osteoarthritis Multiple sclerosis Other anti - inflammatory opportunities Apitox

NYSE American (proposed) : APUS